Human skeletal stem cells isolated in breakthrough discovery
Image: Nissim Benvenisty
- Scientists have isolated skeletal stem cells in adult and fetal bones for the first time.
- These cells could one day help treat damaged bone and cartilage.
- The team was able to grow skeletal stem cells from cells found within liposuctioned fat.
Scientists have isolated human skeletal stem cells from fetal and adult bones, a breakthrough that could lead to better treatments for osteoporosis, fractures, and joint damage.
Discovering stem cells that only produce skeletal structures, like bone and cartilage, has been a decades-long goal for researchers. But it’s been difficult to isolate skeletal stem cells from another type called mesenchymal stem cells, which produce skeletal structures but also fat and muscle.
The team behind the new development, which they described in the journal Cell, had previously discovered skeletal stem cells in mice, a feat they accomplished by creating genetically modified “rainbow mice” whose stem cells had distinct colors. This enabled the researchers to track the course of cells throughout their development and eventually isolate skeletal stem cells.
Isolating skeletal stem cells in humans
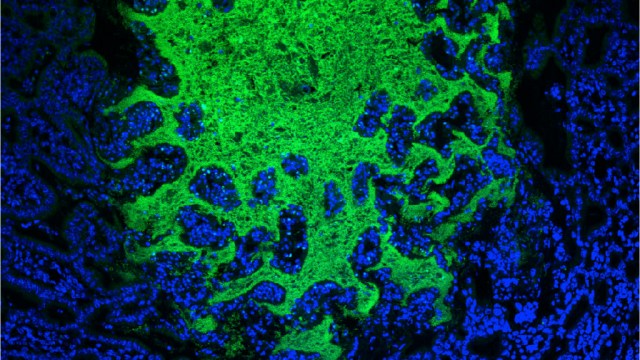
Still, it wasn’t clear these skeletal stem cells existed in humans because we’re vastly more complex than mice. To find out, the team searched fetal bones for stem cells with a genetic signature similar to that of the skeletal stem cells in mice. After isolating these stem cells in lab dishes, they reliably grew into only bone, cartilage, and stroma (essentially, a mix of connective tissue and blood vessels).
The researchers confirmed that these cells were indeed dedicated human skeletal stem cells by isolating the same type of cells from adult human bones, obtained from fragments that had been recently removed during hip surgeries. Again, these stem cells only produced bone, cartilage, and stroma.
What’s more, the researchers were able to produce human skeletal stem cells from stromal cells. As Science reports, they accomplished this by isolating stromal cells and growing them alongside a bone-growth protein.
It turns out there’s an abundant potential source for stromal cells: liposuctioned fat.
“A half-million times a year, U.S. citizens have their fat sucked out and it’s discarded as medical waste,” study author Michael Longaker of Stanford University told Science. “That’s a lot of material we could use to generate skeletal stem cells.”
Although it could take years before these cells are used to treat joint damage or replace broken bones, the discovery provides a proof-of-concept for future treatments of degenerative skeletal conditions like osteoporosis, which affects 44 million Americans.