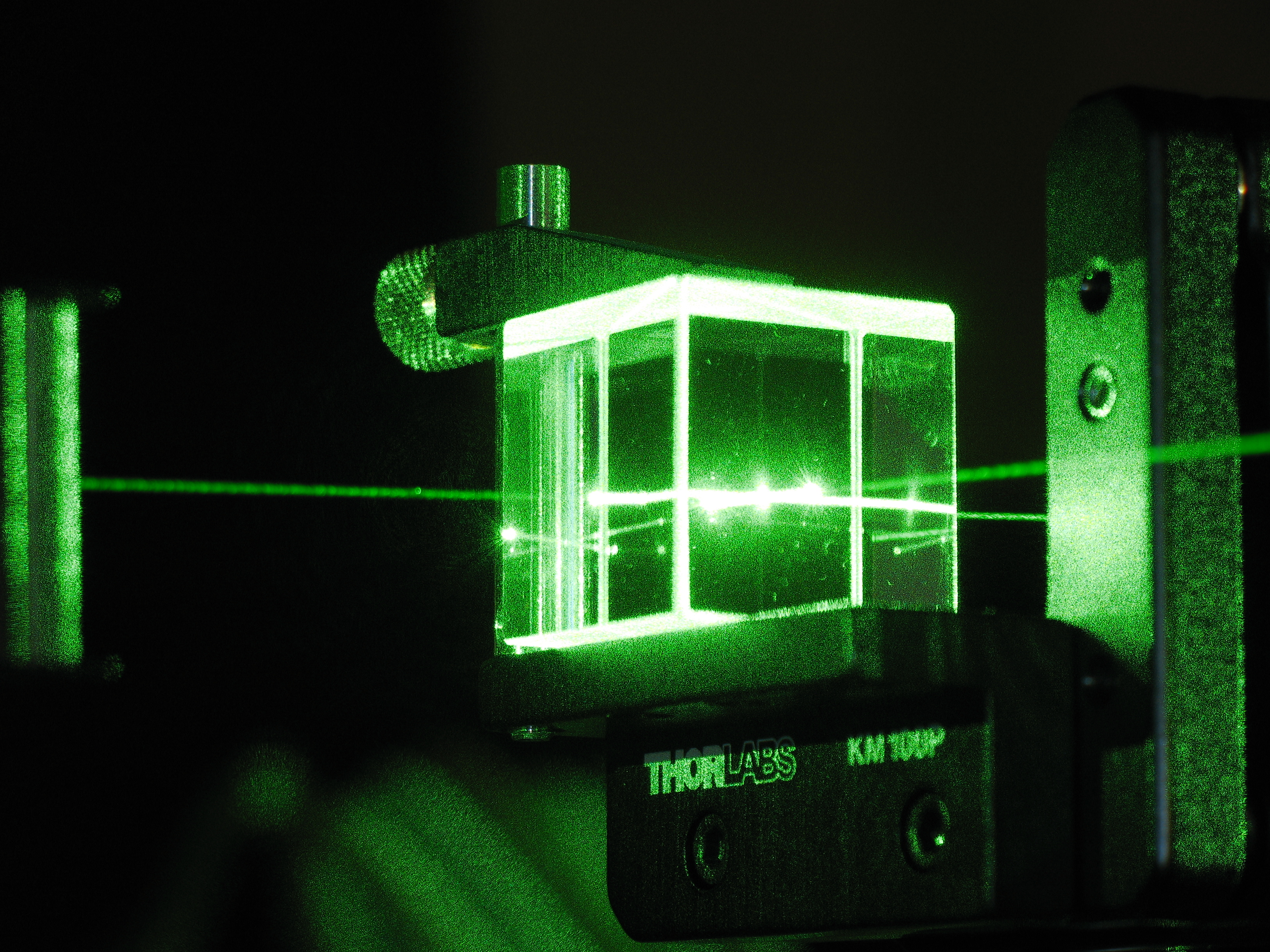
10 quantum myths that need to be busted
- The word quantum makes people think of the fundamental, dual particle-and-wave-like nature of our Universe on the smallest scales of all.
- But this impression has given people the wrong idea: that quantum things are small, that they behave either one way or another, and that entanglement happens faster-than-light.
- The true facts about our quantum reality are much more interesting, and have paved the way for a wide variety of reality-revealing experiments.
For centuries, the laws of physics seemed completely deterministic. If you knew where every particle was, how fast it was moving, and what the forces were between them at any one instant, you could know exactly where they’d be and what they’d be doing at any point in the future. From Newton to Maxwell, the rules that governed the Universe had no built-in, inherent uncertainty to them in any form. Your only limits arose from your limited knowledge, measurements, and calculational power.
All of that changed a little over 100 years ago. From radioactivity to the photoelectric effect to the behavior of light when you passed it through a double slit, we began realizing that under many circumstances, we could only predict the probability that various outcomes would arise as a consequence of the quantum nature of our Universe. But along with this new, counterintuitive picture of reality, many myths and misconceptions have arisen. Here’s the true science behind 10 of them.

1.) Quantum effects only happen on small scales. When we think of quantum effects, we typically think about individual particles (or waves) and the bizarre properties they display. But large-scale, macroscopic effects happen that are inherently quantum in nature.
Conducting metals cooled below a certain temperature become superconductors: where their resistance drops to zero. Building superconducting tracks where magnets levitate above them and travel around them without ever slowing down is a routine student science project these days, built on an inherently quantum effect.
Superfluids can be created on large, macroscopic scales, as can quantum drums that simultaneously do and don’t vibrate. Over the past 25 years, 6 Nobel Prizes have been awarded for various macroscopic quantum phenomena.
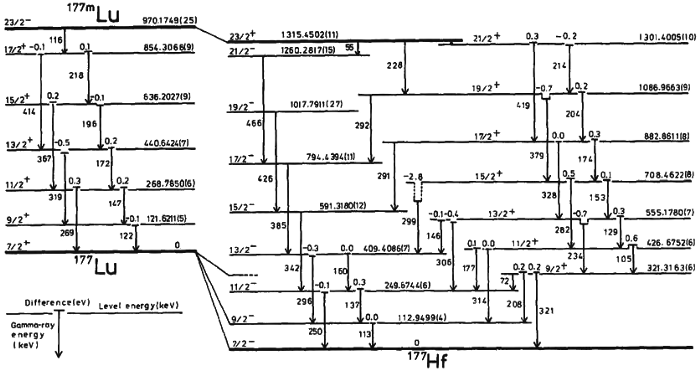
2.) Quantum always means “discrete.” The idea that you can chop up matter (or energy) into individual chunks — or quanta — is an important concept in physics, but it doesn’t fully encompass what it means for something to be “quantum” in nature. For example: consider an atom. Atoms are made of atomic nuclei with electrons bound to them.
Now, think about this question: where is the electron at any moment in time?
Even though the electron is a quantum entity, its position is uncertain until you measure it. Take many atoms and bind them together (such as in a conductor), and you’ll frequently discover that although there are discrete energy levels that the electrons occupy, their positions can literally be anywhere within the conductor. Many quantum effects are continuous in nature, and it’s eminently possible that space and time, at a fundamental, quantum level, are continuous, too.
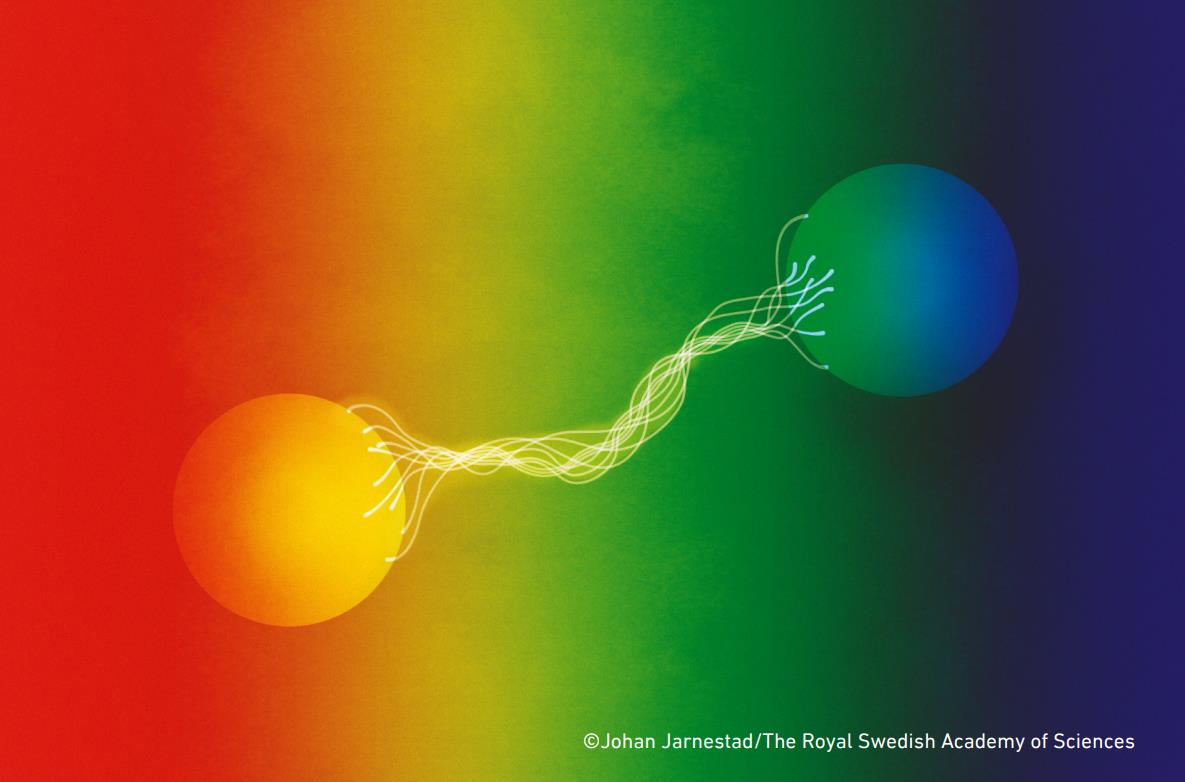
3.) Quantum entanglement allows information to travel faster-than-light. Here’s an experiment we can perform:
- create two entangled particles,
- separate them by a great distance,
- measure certain quantum properties (like the spin) of one particle on your end,
- and you can know some information about the quantum state of other particle instantaneously: faster than the speed of light.
But here’s the thing about this experiment: no information is being transmitted faster than the speed of light. All that’s happening is that by measuring the state of one particle, you are constraining the probable outcomes of the other particle. If someone goes and measures the other particle, they will have no way of knowing that the first particle has been measured and the entanglement has been broken. The only way to determine whether entanglement has been broken or not is to bring the results of both measurements back together again: a process that can only occur at light speed or slower. No information can be passed faster than light; this was proven in a 1993 theorem.
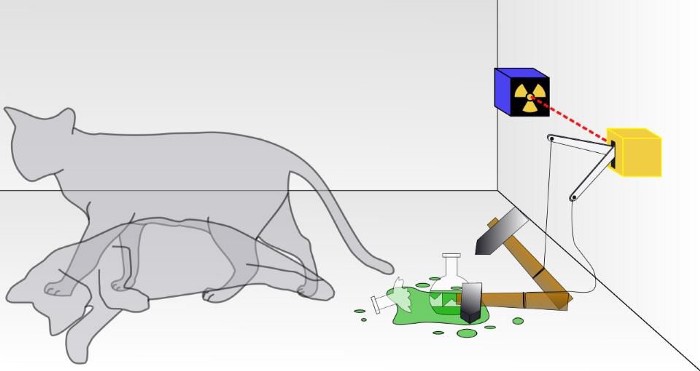
4.) Superposition is fundamental to quantum physics. Imagine you have multiple possible quantum states that a system can be in. Maybe it can be in state “A” with 55% probability, state “B” with 30% probability, and state “C” with 15% probability. Whenever you go to make a measurement, however, you never see a mix of these possible states; you’ll only get a single-state outcome: either it’s “A,” “B,” or “C.”
Superpositions are incredibly useful as intermediate calculational steps to determine what your possible outcomes (and their probabilities) will be, but we can never measure them directly. In addition, superpositions don’t apply to all measurables equally, as you can have a superposition of momenta but not positions or vice versa. Unlike entanglement, which is a fundamental quantum phenomenon, superposition is not quantifiably or universally measurable.
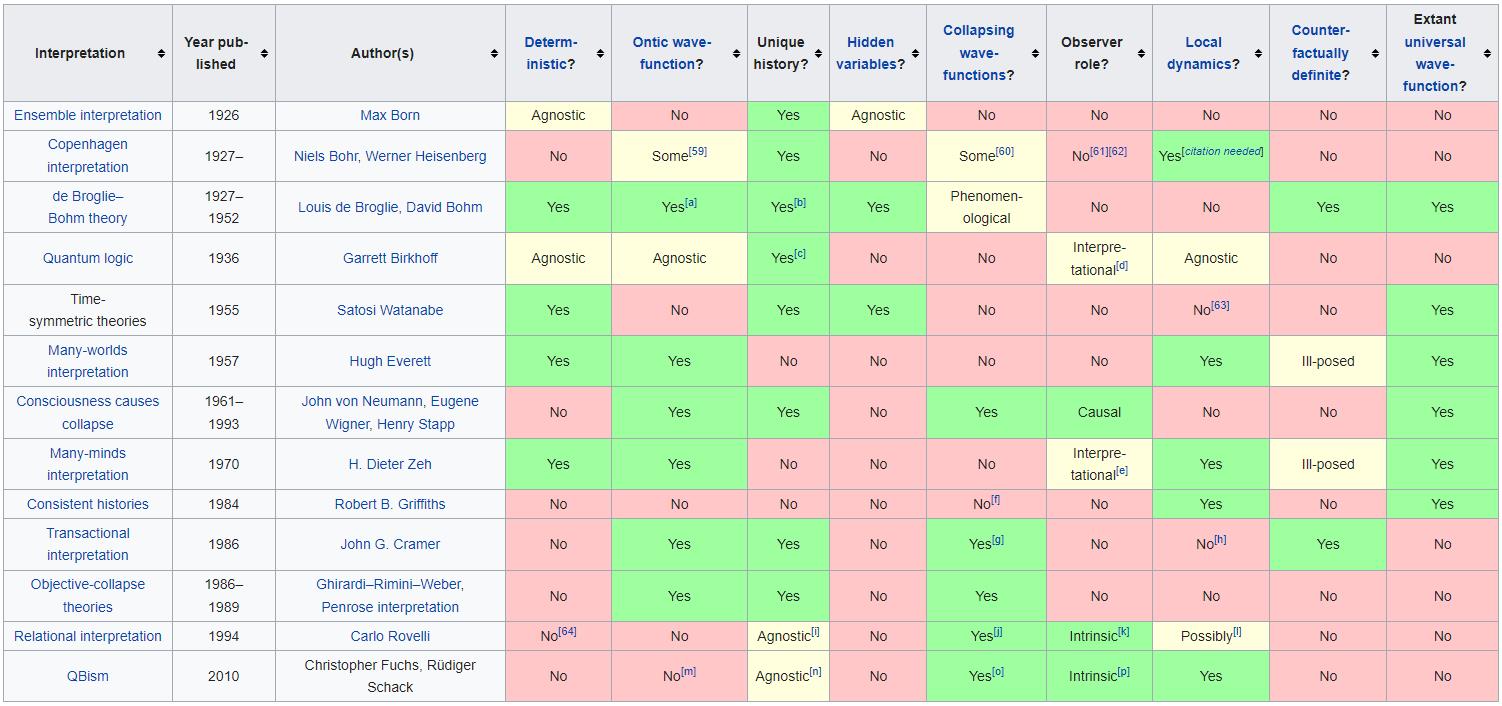
5.) There’s nothing wrong with us all choosing our favorite quantum interpretation. Physics is all about what you can predict, observe, and measure in this Universe. Yet with quantum physics, there are multiple ways to conceive of what’s occurring at a quantum level that all agree equally with experiments. Reality can be:
- a series of quantum wavefunctions that instantaneously “collapse” when a measurement is made,
- an infinite ensemble of quantum waves, where a measurement selects one member of the ensemble,
- a superposition of forward-moving and backward-moving potentials that meet in a “quantum handshake,”
- an infinite number of possible worlds corresponding to the possible outcomes, where we simply occupy one path,
as well as many others. Yet choosing one interpretation over another teaches us nothing except, perhaps, our own human biases. It’s better to learn what we can observe and measure under various conditions, which is physically real, than to prefer an interpretation that has no experimental benefit over any other.
6.) Teleportation is possible, thanks to quantum mechanics. There actually is a real phenomenon known as quantum teleportation, but it most definitively does not mean that it’s physically possible to teleport a physical object from one location to another. If you take two entangled particles and keep one close by while sending the other one to a desired destination, you can teleport the information from the unknown quantum state on one end to the other end.
This has enormous restrictions on it, however, including that it only works for single particles and that only information about an indeterminate quantum state, not any physical matter, can be teleported. Even if you could scale this up to transmit the quantum information that encodes an entire human being, transferring information is not the same as transferring matter: you cannot teleport a human, ever, with quantum teleportation.
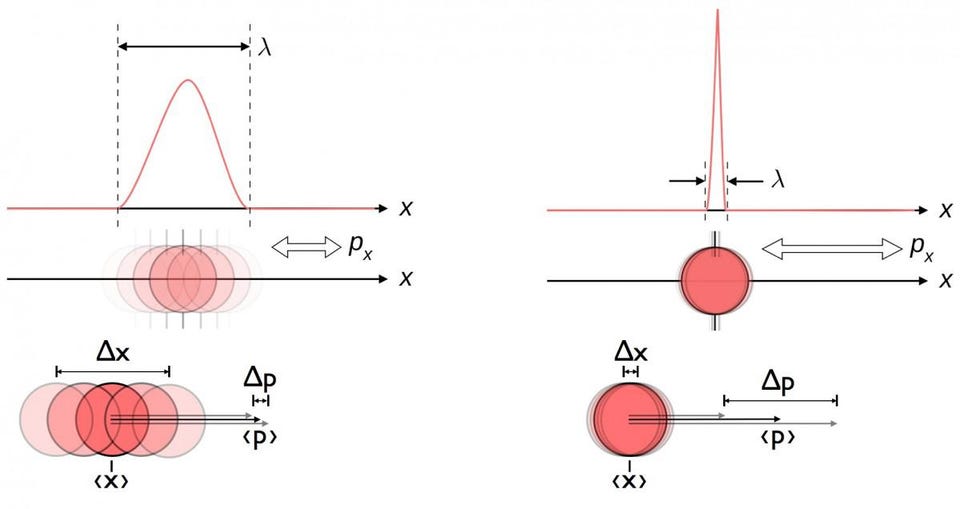
7.) Everything is uncertain in a quantum Universe. Some things are uncertain, but many things are extremely well-defined and well-known in a quantum Universe. If you take an electron, for example, you cannot know:
- its position and its momentum,
- or its angular momentum in multiple, mutually perpendicular directions,
exactly and simultaneously under any circumstances. But some things about the electron can be known exactly! We can know its rest mass, its electric charge, or its lifetime (which appears to be infinite) with exact certainty.
The only things that are uncertain in quantum physics are pairs of physical quantities that have a specific relationship between them: that are pairs of conjugate variables. This is why there are uncertainty relations between energy and time, voltage and free charge, or angular momentum and angular position. While many pairs of quantities have an inherent uncertainty between them, many quantities are still known exactly.
8.) Every particle of the same type has the same mass. If you could take two identical particles — like two protons or two electrons — and put them on a perfectly accurate scale, they’d always have the same exact mass as one another. But that’s only because protons and electrons are stable particles with infinite lifetimes.
If you instead took unstable particles that decayed after a short while — such as two top quarks or two Higgs bosons — and put them on a perfectly accurate scale, you wouldn’t get the same values. This is because there’s an inherent uncertainty between energy and time: if a particle only lives for a finite amount of time, then there’s an inherent uncertainty in the amount of energy (and hence, from E = mc², rest mass) that the particle has. In particle physics, we call this a particle’s “width,” and it can lead to a particle’s inherent mass being uncertain by up to a few percent.

9.) Einstein himself denied quantum mechanics. It’s true that Einstein had a famous quote about how, “God does not play dice with the Universe.” But arguing against a fundamental randomness inherent to quantum mechanics — which is what the context of that quote was about — is arguing about how to interpret quantum mechanics, not an argument against quantum mechanics itself.
In fact, the nature of Einstein’s argument was that there might be more to the Universe than we can presently observe, and if we could understand the rules we have not yet uncovered, perhaps what appears to be randomness to us here might reveal a deeper, non-random truth. Although this position has not yielded useful results, explorations of the fundamentals of quantum physics continues to be an active area of research, successfully ruling out a number of interpretations involving “hidden variables” present in the Universe.
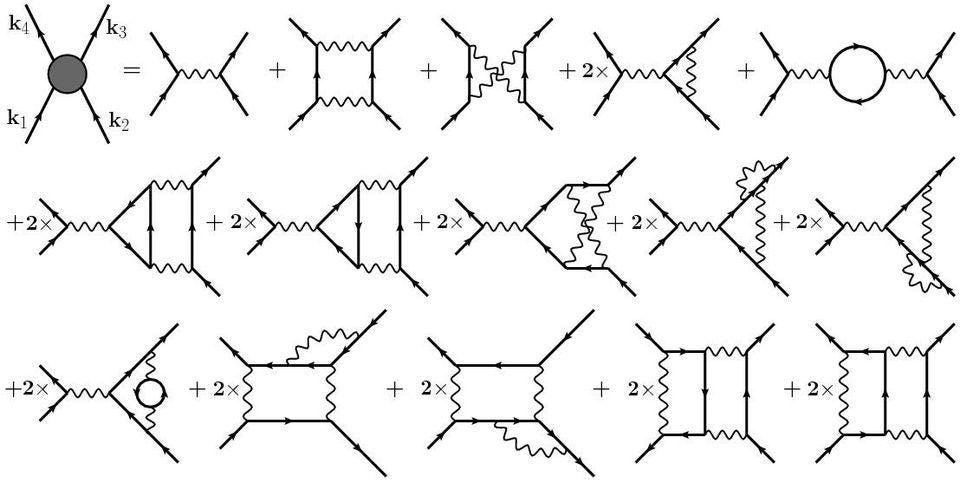
10.) Exchanges of particles in quantum field theory completely describe our Universe. This is the “dirty little secret” of quantum field theory that physicists learn in graduate school: the technique we most commonly use for calculating the interactions between any two quantum particles. We visualize them as particles being exchanged between those two quanta, along with all possible further exchanges that could occur as intermediate steps.
If you could extrapolate this to all possible interactions — to what scientists call arbitrary loop-orders — you’d wind up with nonsense. This technique is only an approximation: an asymptotic, non-convergent series which breaks down past a certain number of terms. It’s an incredibly useful picture, but fundamentally incomplete. The idea of virtual particle exchanges is compelling and intuitive, but is unlikely to be the final answer.