The 6 strongest materials on Earth are harder than diamonds

- The bond structure of carbon, as an atom, has some special properties that allow it to form extremely hard materials when bound together in a lattice.
- Although diamonds, crystalline structures known since antiquity, are extraordinarily hard, they’re not the hardest possible configuration, and in fact some non-carbon-containing compounds can be harder.
- You might think that the hardest possible materials could only occur in the lab, but the Universe has ways of surprising us, even on this front.
Carbon is one of the most fascinating elements in all of nature, with chemical and physical properties unlike any other element. With just six protons in its nucleus, it’s the lightest abundant element capable of forming a slew of complex bonds. All known forms of life are carbon-based, as its atomic properties enable it to link with up to four other atoms at a time, from the simplest such structure possible (methane) to incredibly rich molecules containing trillions of atoms or more. The possible geometries of those bonds also enable carbon to self-assemble, particularly under high pressures, into a stable crystal lattice.
If the conditions are just right, with impurity-free carbon and the needed pressures and temperatures, carbon atoms can form a solid, ultra-hard structure known as a diamond. Although diamonds are commonly known as “the hardest material in the world,” there are actually six materials that are harder. Diamonds are still one of the hardest naturally occurring (and, perhaps surprisingly, quite abundant) materials on Earth, and yet these six materials all have diamonds beat.

Credit: I. Agnarsson, M. Kuntner, and T.A. Blackledge, PLOS ONE, 2010
Honorable mentions
There are three terrestrial materials that aren’t quite as hard as diamond, but are still remarkably interesting for their strength in a variety of fashions. With the advent of nanotechnology — alongside the development of nanoscale understandings of modern materials — we now recognize that there are many different metrics to evaluate physically interesting and extreme materials. For example, hardness, scratch resistance, durability (as many very hard materials are also brittle), and the ability to withstand extreme environmental stresses (such as pressures or temperatures) all factor into how impressive a material can be.
On the biological side, spider silk is notorious as the toughest material produced by a plant, animal, or fungus. With a higher strength-to-weight ratio than most conventional industrial materials or alloys, like aluminum or steel, spider silk is also remarkably thin and sticky. Of all the spiders in the world, Darwin’s bark spiders produce the toughest silk: ten times stronger than kevlar. It’s so thin and light that approximately a pound (454 grams) of Darwin’s bark spider silk would compose a strand long enough to trace out the circumference (~40,000 km) of the entire planet.
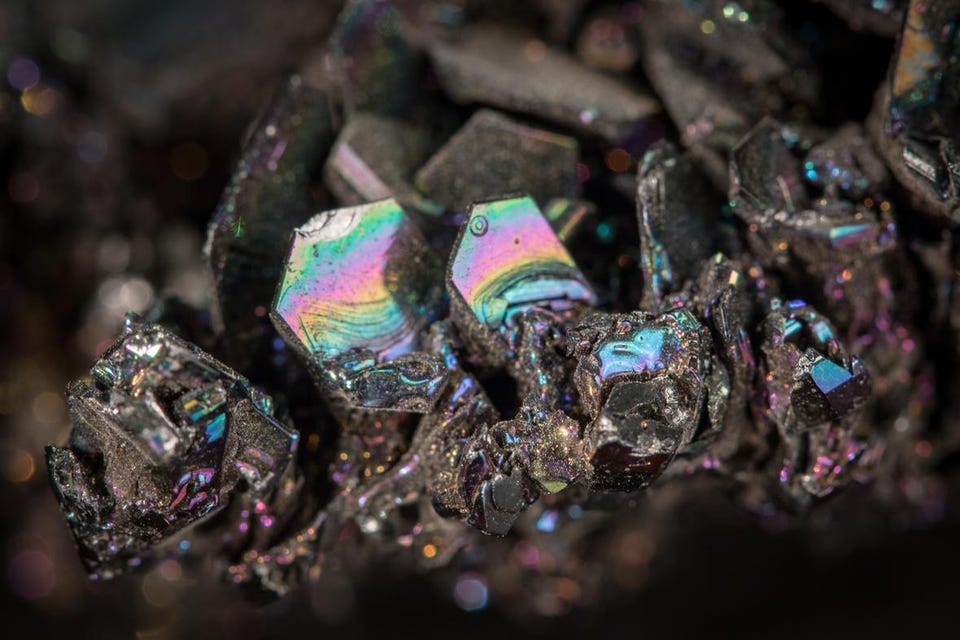
Credit: Scott Horvath/USGS
A naturally occurring mineral, silicon carbide — found naturally in the form of moissanite — is only slightly lower on the Mohs hardness scale than diamonds. (And it’s still substantially harder than the silk of any known spider.) A chemical mix of silicon and carbon, which occupy the same family in the periodic table as one another, silicon carbide grains have been mass produced since 1893. They can be bonded together through a high-pressure but low-temperature process known as sintering to create extremely hard ceramic materials.
These materials are not only useful in a wide variety of applications that take advantage of hardness, such as car brakes and clutches, plates in bulletproof vests, and even battle armor suitable for tanks, but also have incredibly useful semiconductor properties for use in electronic and computer technologies.
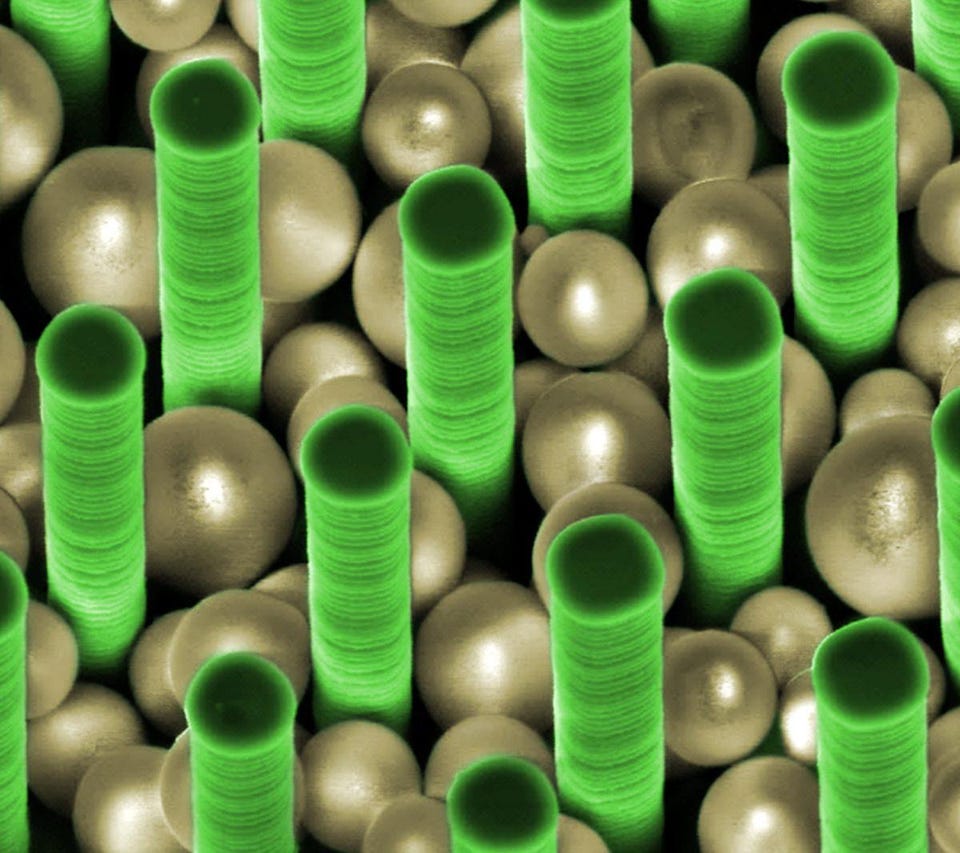
Credit: Oak Ridge National Laboratories/flickr
Tiny silica spheres, from 50 nanometers in diameter down to just 2 nanometers, were created for the first time back in 1999 at the Department of Energy’s Sandia National Laboratories. Remarkably, these nanospheres are hollow: they self-assemble into spheres, and they can even nest inside one another, all while remaining the stiffest (or lowest-deformity) material known to humankind, while still being only slightly less hard than diamonds on the Mohs scale.
Self-assembly is an incredibly powerful tool in nature, but biological materials are almost always weaker when compared with synthetic ones. These self-assembling nanoparticles have a wide variety of commercial uses; they could be used to create custom materials with applications from better water purifiers to more efficient solar cells, from faster catalysts to next-generation electronics. The dream technology of these self-assembling nanospheres, though, is printable body armor, which would then be completely customizable to the user’s specifications.

Credit: Sararwut Jaimassiri/Shutterstock
The #7 hardest material: diamonds
Diamonds, of course, are harder than every one of these aforementioned materials, but still clock in at only #7 on the all-time list of hardest materials ever found, synthesized, or otherwise created on Earth. Despite the fact that they’ve been surpassed by both other natural (but rare) materials as well as synthetic, human-made ones, they do still hold one important record: that of the most scratch-resistant material known to humanity.
While other materials may rank higher on the Mohs hardness scale than diamonds, they’re all easier to scratch than diamonds are. (And, consequently, can be scratched or otherwise damaged through contact with a diamond.) Metals like titanium are far less scratch-resistant, and even extremely hard ceramics or tungsten carbide cannot compete with diamonds in terms of both hardness and scratch-resistance. Other crystals that are known for their extreme hardness, such as rubies or sapphires, still fall short of diamonds, as their impurities render them so.
However, there are six known materials that have surpassed even the vaunted diamond in terms of pure hardness.
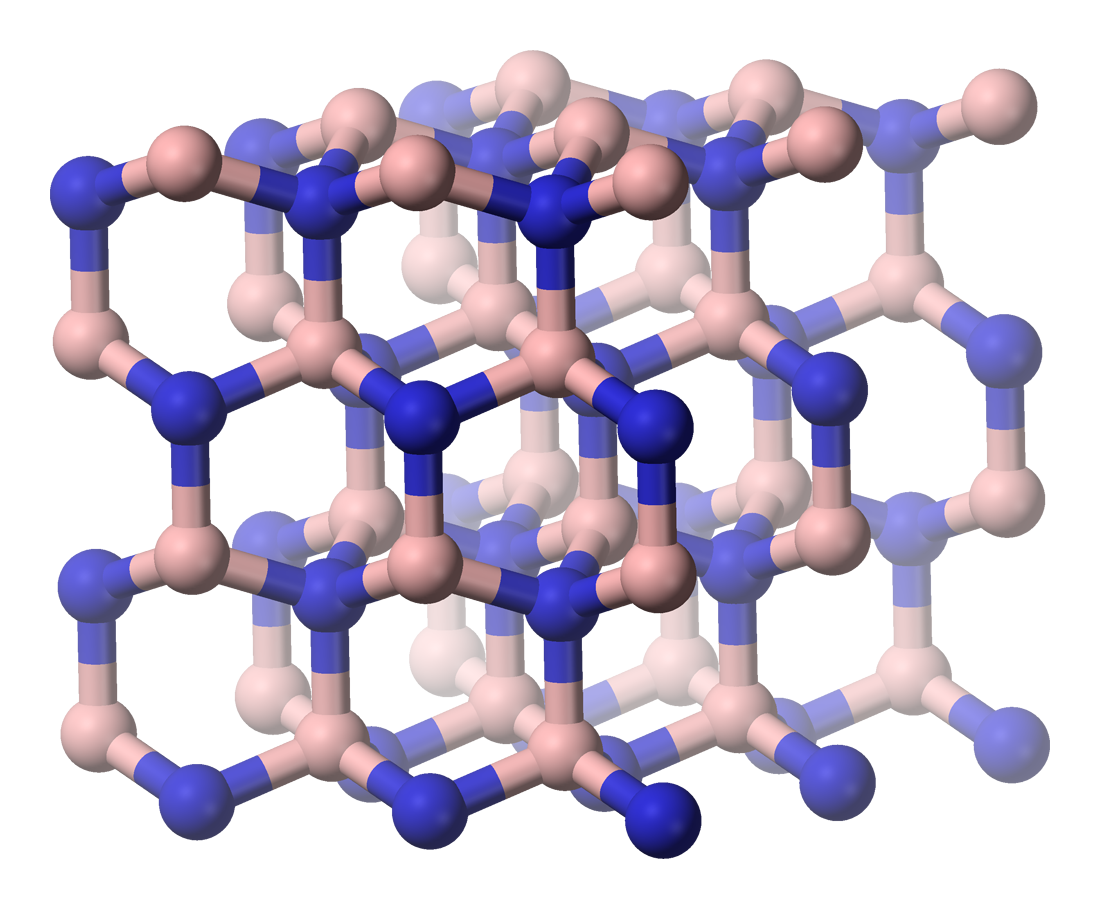
Credit: Benjah-bmm27/public domain
The #6 hardest material: Wurtzite boron nitride
Although carbon may be the most famous material for building crystal lattices out of, it’s far from the only one. You can assemble a crystal out of a number of other atoms or compounds, with one of them being boron nitride (BN), where the 5th and 7th elements on the periodic table come together to allow a variety of possible configurations. It can be amorphous (non-crystalline), hexagonal (similar to graphite), cubic (similar to diamond, but slightly weaker), and can also take on the wurtzite form, which lands it on this list.
The final one of these forms is extremely rare, but it’s also extremely hard. Formed during volcanic eruptions, it’s only ever been discovered in minute quantities, quantities that are too small to actually test its hardness properties directly, via experiment. However, it forms a different kind of crystal lattice — a tetrahedral one instead of a face-centered cubic one — that is 18% harder than diamond, according to the most recent simulations, placing it in 6th on this list.
Credit: H. Ohfugi et al., Nature Scientific Reports, 2015
The #5 hardest material: Lonsdaleite
Imagine what would happen if you had a meteor that was full of carbon, and therefore contained substantial quantities of the carbon-rich mineral graphite, that hurtled through our atmosphere and wound up colliding with the surface of planet Earth. While you might envision a falling meteor as an incredibly hot body, it’s only the outer layers that become hot; the insides remain cool for most (or even, potentially, all) of its journey toward — and eventual impact with — Earth.
Upon impact with Earth’s surface, however, the pressures inside the meteorite (which is what meteors become when they make it down to the ground) now become larger than any other natural process on our planet’s surface. These incredibly large pressures cause the graphite to compress into a crystalline structure. Under these conditions, the crystal won’t possess the cubic lattice structure of a diamond, however, but rather a hexagonal lattice, which can actually achieve hardnesses that are 58% greater than what diamonds achieve. While real, recovered examples of lonsdaleite from meteorites contain sufficient impurities to make them actually softer than diamonds, an impurity-free graphite meteorite striking the Earth would undoubtedly produce material harder than any terrestrial diamond.

Credit: Justsail/Wikimedia Commons
The #4 hardest material: Dyneema
From here on out, we leave the realm of naturally occurring substances behind, with only one notable exception yet to come. Dyneema, a thermoplastic polyethylene polymer, is unusual for having an extraordinarily high molecular weight. Most molecules that we know of are chains of atoms with a few thousand atomic mass units (protons and/or neutrons) in total. But UHMWPE (for ultra-high-molecular-weight polyethylene) has extremely long chains, with a molecular mass in the millions of atomic mass units per molecule within the larger macroscopic material.
With very long chains for their polymers, the intermolecular interactions are substantially strengthened, creating a very tough material. It’s so tough, in fact, that it has the highest impact strength of any known thermoplastic. It has been called the strongest fiber in the world and outperforms all other mooring and tow ropes. Despite being lighter than water in terms of density (which means that it floats), it can stop bullets and has 15 times the material strength of a comparable amount of steel. Although it’s bendable, it is extremely hard, scratch-and-fray resistant, and is incredibly difficult to break even when amazingly strong forces are applied to it.
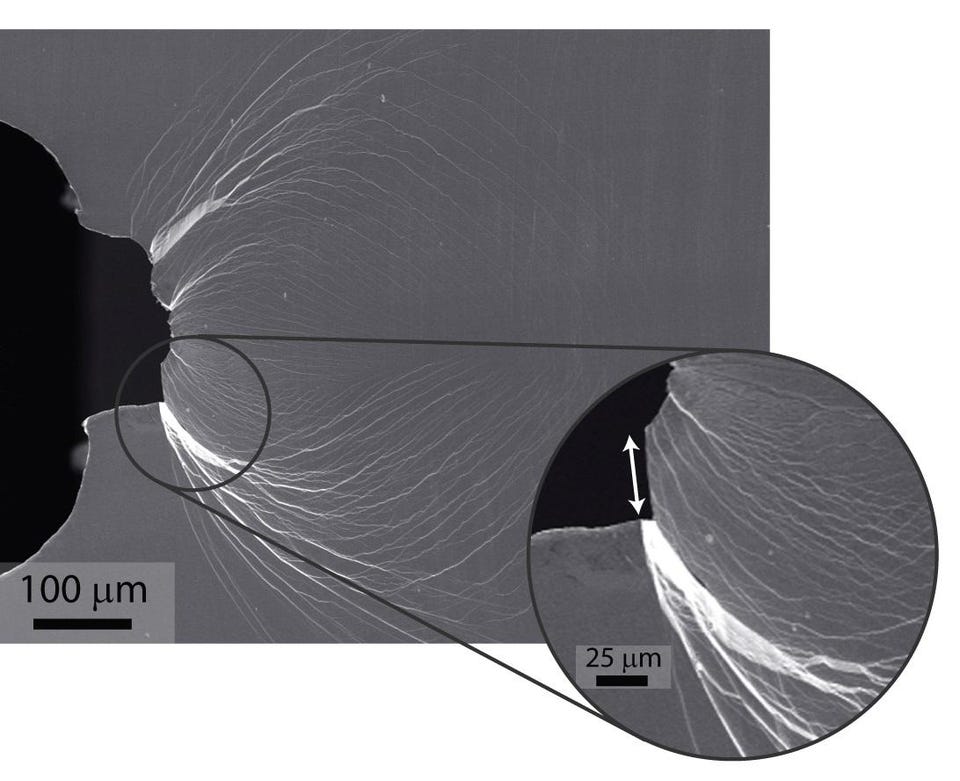
Credit: R. Ritchie and M. Demetriou
The #3 hardest material: Palladium microalloy glass
It’s important to recognize that there are two important properties, in addition to “hardness,” that all physical materials possess:
- strength, which is how much force it can withstand before it deforms,
- and toughness, which is how much energy it takes to break or fracture it.
Most ceramics are strong but not tough, shattering with vice grips or even when dropped from only a modest height. Elastic materials, like rubber, can hold a lot of energy but are easily deformable, and not strong at all.
Most glassy materials are brittle: strong but not particularly tough. Even reinforced glass, like Pyrex or Gorilla Glass, isn’t particularly tough on the scale of materials. But in 2011, researchers developed a new microalloy glass featuring five elements (phosphorus, silicon, germanium, silver, and palladium), where the palladium provides a pathway for forming shear bands, allowing the glass to plastically deform rather than crack. This means that this palladium microalloy glass is both strong and tough, in addition to being very hard.
It defeats all types of steel, as well as anything lower on this list, for its combination of both strength and toughness. It is the hardest material known that doesn’t include any carbon atoms.

Credit: NanoLab, Inc.
The #2 hardest material: Buckypaper
It is well-known since the late 20th-century that there’s a form of carbon that’s even harder than diamonds: carbon nanotubes. By binding carbon together into a hexagonal shape, it can hold a rigid cylindrical-shaped structure more stably than any other structure known to humankind. If you take an aggregate of carbon nanotubes and create a macroscopic sheet of them, the thin sheet of them that you will have created becomes a material with its own name: buckypaper.
Named after Buckminster Fuller, this structure is matched in hardness by the related buckyball, which is 60 carbon atoms bound together. Buckyballs do occur in nature and are found in certain naturally occurring environments in interstellar space. Although buckyballs (like individual carbon nanotubes) may have applications to nanotechnology, they aren’t generally scalable to macroscopic scales, and hence aren’t deserving of their own spot on this list of “hardest materials.”
For buckypaper, however, while each individual nanotube is only between 2 and 4 nanometers across, these individually strong and tough structures can be bound together into large, macroscopic sheets. It’s only 10% the weight of steel but has hundreds of times the strength. It’s fireproof, extremely thermally conductive, possesses tremendous electromagnetic shielding properties, and will no doubt lead to materials science, electronics, military, and even biological applications. However, buckypaper has a limitation: it cannot be made 100% out of carbon nanotubes, which is perhaps what keeps it from equaling the material that reaches the top spot on this list.
Credit: AlexanderAIUS/Core-Materials/flickr
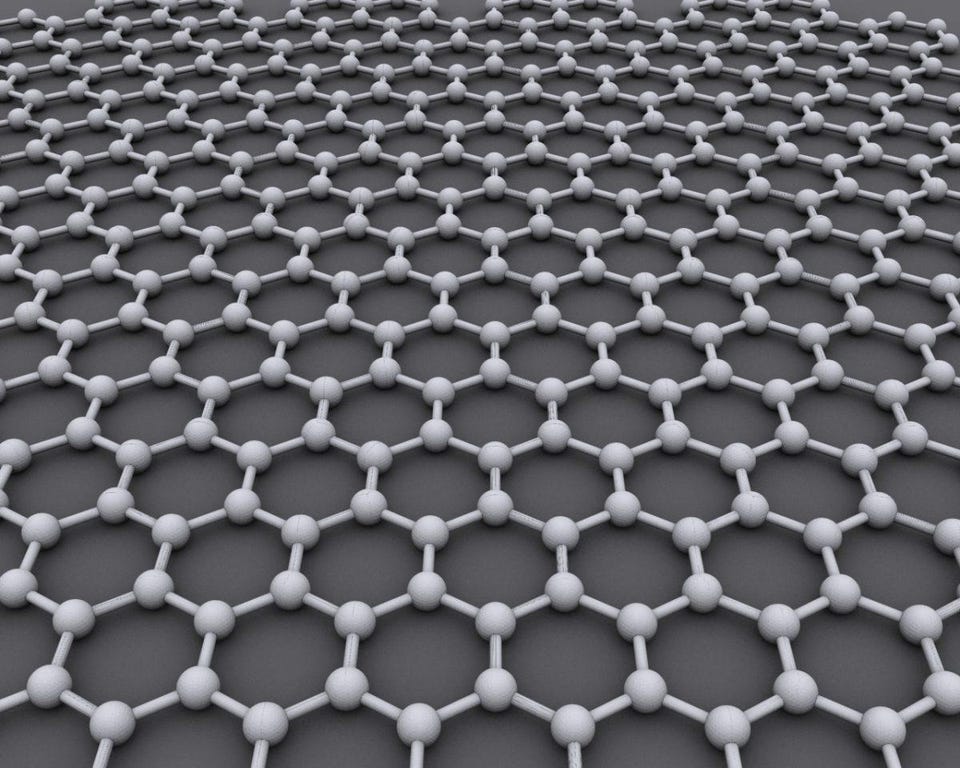
The #1 hardest material: Graphene
At last, it’s the hardest material of all: a hexagonal carbon lattice that is only a single atom thick. A sheet of graphene is arguably the most revolutionary material to be developed and utilized in the 21st century. It is the basic structural element of carbon nanotubes themselves (which is a single graphene sheet folded into a thin cylinder), and the applications for graphene are growing continuously over time. Graphene became a billion-dollar industry for the first time in 2024, and is expected to grow to triple that value within the next five years.
In proportion to its thickness, graphene is far and away the strongest material known, while also being an extraordinary conductor of both heat and electricity and nearly 100% transparent to light, to boot. The 2010 Nobel Prize in Physics went to Andre Geim and Konstantin Novoselov for groundbreaking experiments involving graphene, and the commercial applications continue to pour in. To date, graphene is the thinnest material known, and the mere six year gap between Geim and Novoselov’s work and their Nobel award is one of the shortest in the history of physics.
Credit: Workbit/Wikimedia Commons
The quest to make materials more extreme in a variety of ways, including to make them:
- harder,
- stronger,
- more scratch-resistant,
- lighter,
- tougher,
- etc.,
is probably never going to end. If humanity can push the frontiers of the materials available to us farther than ever before, the applications for what becomes feasible — and eventually practical — can only expand. Generations ago, the idea of microelectronics, transistors, or the capacity to manipulate individual atoms was exclusive to the realm of science-fiction. Today, they’re so common that we take all of these technologies, and the associated science that underlies them, completely for granted.
As we hurtle full-force into the age of nanotechnology, materials such as the ones described here become increasingly more important to our quality of life, as well as more ubiquitous throughout our modern society. It’s a wonderful thing to live in a civilization where diamonds are no longer the hardest known material; the scientific advances we continue to make offer benefits to society as a whole. As the 21st century continues to unfold, let’s hope that we will all get to see what becomes possible with the advent of these new materials.
This article was originally published in March of 2022. It was updated in 2025.





