Is the new ‘right-to-try’ law libertarian quackery or lifesaving hope?

President Donald Trump signed a bill on Wednesday that makes it easier for terminally ill patients to access treatments that have yet to receive FDA approval.
Dubbed “right to try,” the law allows terminally ill patients and their doctors to work directly with drug manufacturers to access drug treatments that “have passed Phase 1 of the Food and Drug Administration’s approval process” but have not passed the remaining two phases, or a reviewal process.
“Thousands of terminally ill Americans will finally have hope, and the fighting chance, and I think it’s going to better than a chance, that they will be cured, they will be helped, and be able to be with their families for a long time, or maybe just for a longer time,” Trump said Wednesday at the White House, surrounded by terminally ill patients and their families, adding that they’ll finally have “the right to try.”
Right-to-try legislation has already passed in some 38 states, but passing it on a federal removes legal roadblocks that made it difficult for patients and drug manufacturers to transact across state lines.
Supporters of the bill have noted the lengthy FDA approval process requires time that dying patients simply don’t have. Others argue that shutting out the FDA is dangerous.
“FDA oversight of access to experimental treatments exists for a reason — it protects patients from potential snake oil salesmen or from experimental treatments that might do more harm than good,” said Rep. Frank Pallone Jr. (D-N.J.), ranking member of the House Energy and Commerce Committee.
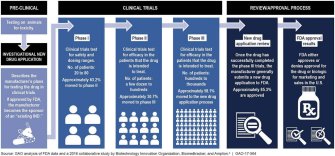
A letter drafted and signed by more than 300 medical professionals notes that there’s already “compassionate use” laws that allow physicians to submit Emergency Investigational New Drug applications to the FDA on behalf of patients to access drug treatments that have yet to be approved. Right-to-try supporters have argued that it can take up to 100 hours for physicians to fill out these applications, but the FDA says it’s streamlined the process down to 45 minutes.
The letter, which was sent to the House Committee on Energy and Commerce in February, points out that the FDA approves 99.7 percent of these applications.
“Because the FDA is not the obstacle to patient access to investigational drugs and plays a vital role in ensuring proper patient safeguards are in place, we implore the Committee to not pass legislation that would remove the FDA from the initial authorization process for accessing an investigational therapy outside of a clinical trial,” the letter reads.
Most drugs that begin FDA screening never get approved, as Sarah Karlin-Smith wrote for Politico:
“The vast majority of experimental drugs ultimately fail: FDA estimates that for every 100 drugs that enter the first phase of human testing, only five to seven will eventually get approval. The other 95 percent either didn’t work, or had risks that outweighed their benefits, and any patients who took them on an ‘experimental’ basis would have suffered pointlessly.”
On a deeper level, right-to-try can be seen as another battle in the war to roll back government regulation. The legislation Trump signed Wednesday was first drafted by the Goldwater Institute, a libertarian think tank that’s also successfully pushed, in several states, legislation that allows drug makers to promote so-called “off-label” uses on medicine.
It seems clear that the Goldwater Institute wants to minimize the FDA’s regulatory power in a broad sense, in such a way that patients, not just the terminally ill, could someday start asking why they can’t have immediate access to any drug treatment they wish to try.
“The ultimate aim is a kind of libertarian paradise of deregulation,” Daniel Carpenter, a professor of government at Harvard and author of a book on the FDA’s history, told The Nation.
The Goldwater Institute and its supporters, according to Carpenter, are trying to reframe the conversation on drug regulation to one about rights rather than safety. That’s a legal metaphor that’s “deeply resonant” in American politics, he said, adding that passing the bill would amount to ideology trumping science.
Dr. Scott Gottlieb, the commissioner of the FDA, had expressed concern over right-to-try legislation, but wrote on Twitter earlier this month that he stands “ready to implement it in a way that achieves Congress’ intent to promote access and protect patients; and build on#FDA’s longstanding commitment to these important goals#RTT.”





