Not just light: Everything is a wave, including you

- Quantum physics has redefined our understanding of matter.
- In the 1920s, the wave-particle duality of light was extended to include all material objects, from electrons to you.
- Cutting-edge experiments now explore how biological macromolecules can behave as both particle and wave.
In 1905, the 26-year-old Albert Einstein proposed something quite outrageous: that light could be both wave or particle. This idea is just as weird as it sounds. How could something be two things that are so different? A particle is small and confined to a tiny space, while a wave is something that spreads out. Particles hit one another and scatter about. Waves refract and diffract. They add on or cancel each other out in superpositions. These are very different behaviors.
Hidden in translation
The problem with this wave-particle duality is that language has issues accommodating both behaviors coming from the same object. After all, language is built of our experiences and emotions, of the things we see and feel. We do not directly see or feel photons. We probe into their nature with experimental set-ups, collecting information through monitors, counters, and the like.
The photons’ dual behavior emerges as a response to how we set up our experiment. If we have light passing through narrow slits, it will diffract like a wave. If it collides with electrons, it will scatter like a particle. So, in a way, it is our experiment, the question we are asking, that determines the physical nature of light. This introduces a new element into physics: the observer’s interaction with the observed. In more extreme interpretations, we could almost say that the intention of the experimenter determines the physical nature of what is being observed — that the mind determines physical reality. That’s really out there, but what we can say for sure is that light responds to the question we are asking in different ways. In a sense, light is both wave and particle, and it is neither.
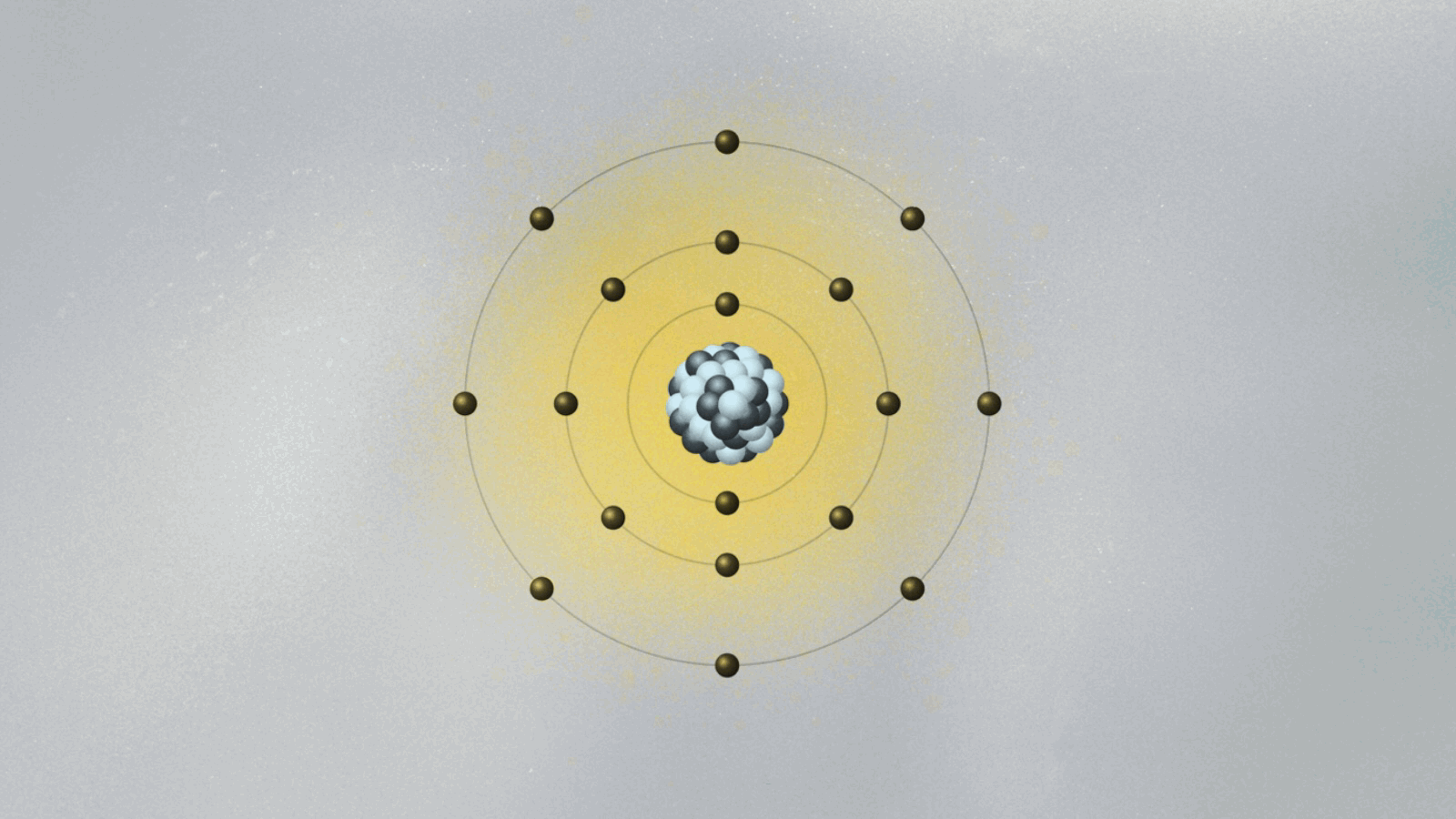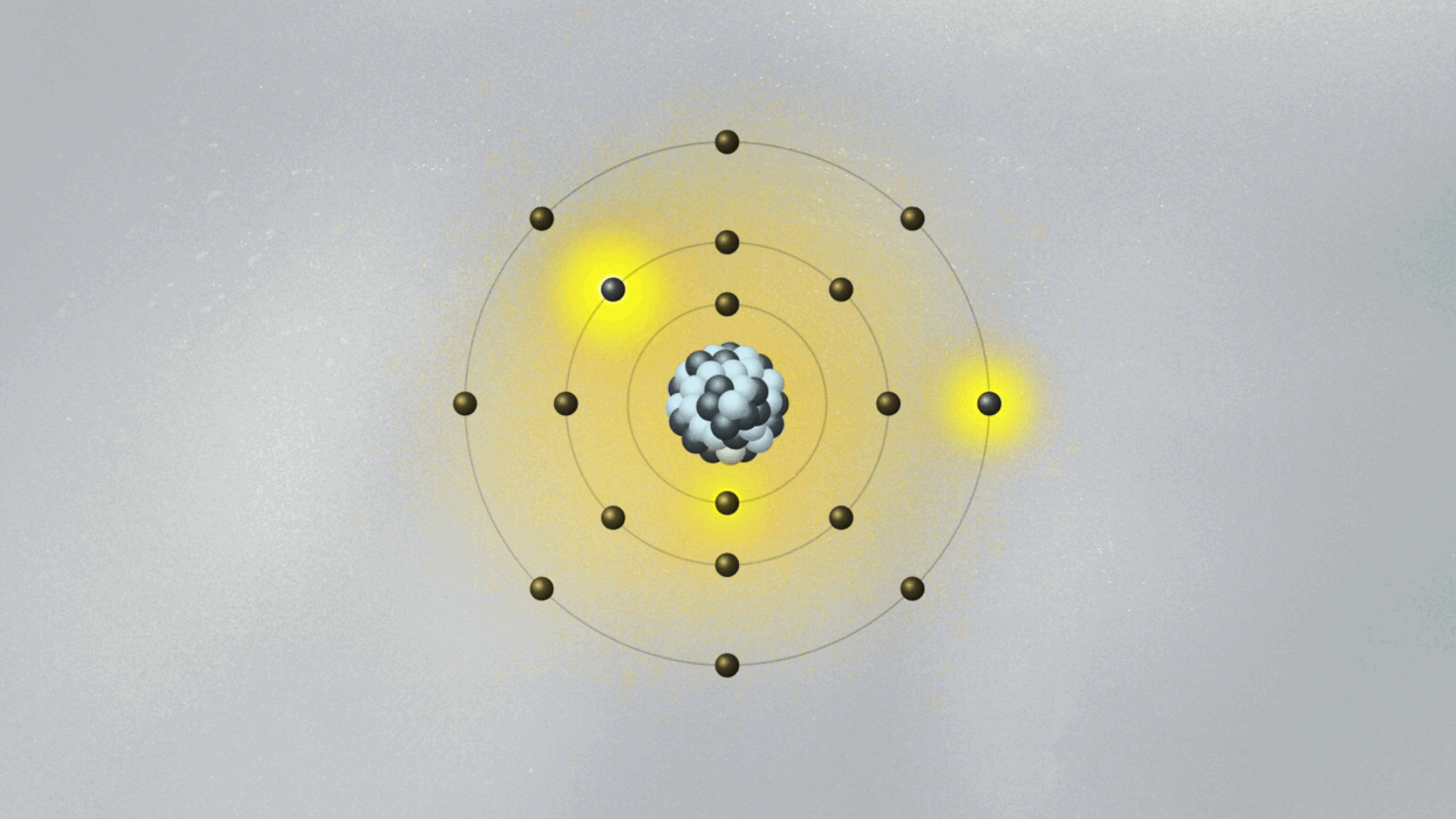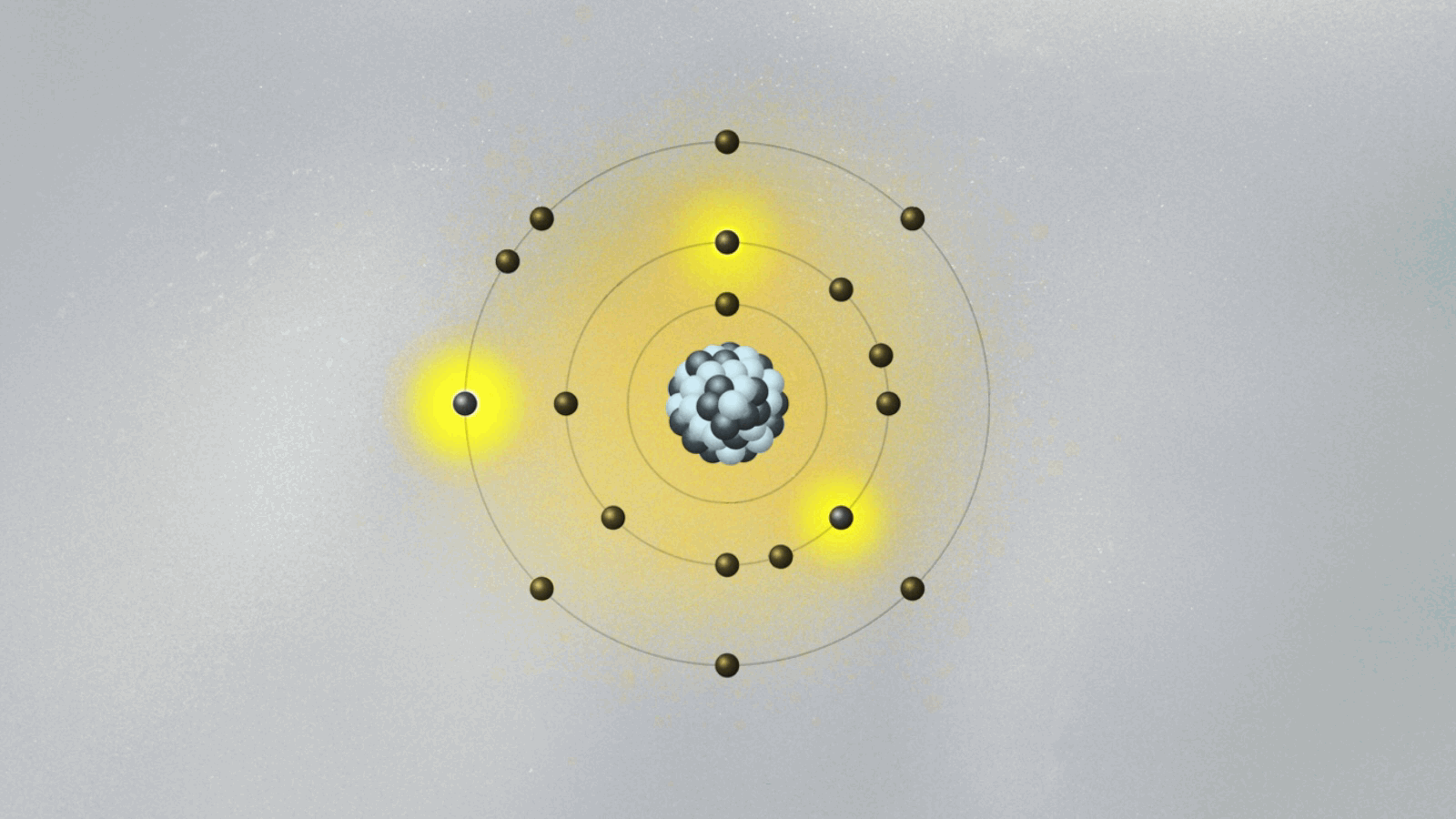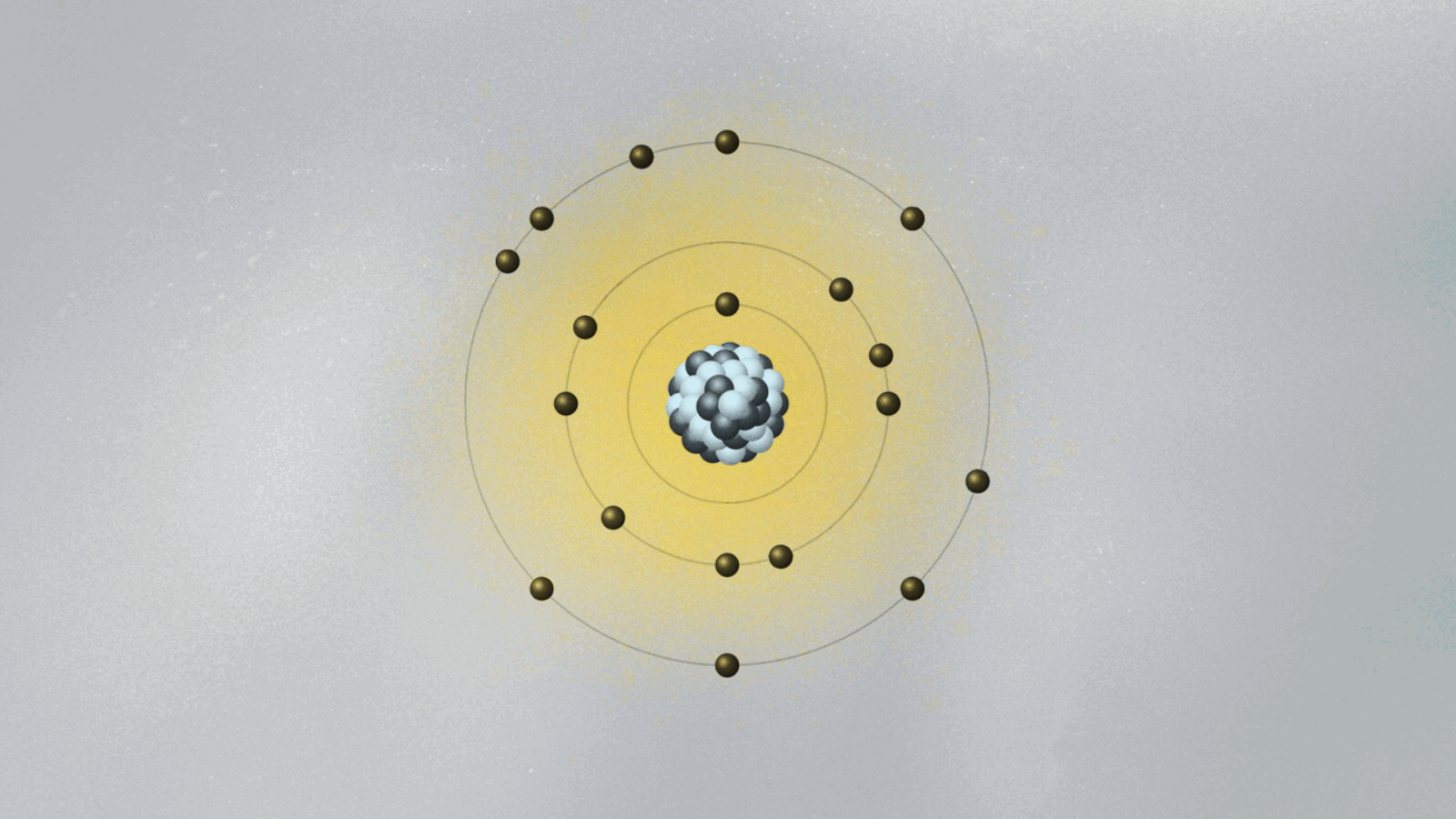
This brings us to Bohr’s model of the atom, which we discussed a couple of weeks back. His model pins electrons orbiting the atomic nucleus to specific orbits. The electron can only be in one of these orbits, as if it is set on a train track. It can jump between orbits, but it cannot be in between them. How does that work, exactly? To Bohr, it was an open question. The answer came from a remarkable feat of physical intuition, and it sparked a revolution in our understanding of the world.
The wave nature of a baseball
In 1924, Louis de Broglie, a historian turned physicist, showed quite spectacularly that the electron’s step-like orbits in Bohr’s atomic model are easily understood if the electron is pictured as consisting of standing waves surrounding the nucleus. These are waves much like the ones we see when we shake a rope that is attached at the other end. In the case of the rope, the standing wave pattern appears due to the constructive and destructive interference between waves going and coming back along the rope. For the electron, the standing waves appear for the same reason, but now the electron wave closes on itself like an ouroboros, the mythic serpent that swallows its own tail. When we shake our rope more vigorously, the pattern of standing waves displays more peaks. An electron at higher orbits corresponds to a standing wave with more peaks.
With Einstein’s enthusiastic support, de Broglie boldly extended the notion of wave-particle duality from light to electrons and, by extension, to every moving material object. Not only light, but matter of any kind was associated with waves.
De Broglie offered a formula known as de Broglie wavelength to compute the wavelength of any matter with mass m moving at velocity v. He associated wavelength λ to m and v — and thus to momentum p = mv — according to the relation λ = h/p, where h is Planck’s constant. The formula can be refined for objects moving close to the speed of light.
As an example, a baseball moving at 70 km per hour has an associated de Broglie wavelength of about 22 billionths of a trillionth of a trillionth of a centimeter (or 2.2 x 10-32 cm). Clearly, not much is waving there, and we are justified in picturing the baseball as a solid object. In contrast, an electron moving at one-tenth the speed of light has a wavelength about half the size of a hydrogen atom (more precisely, half the size of the most probable distance between an atomic nucleus and an electron at its lowest energy state).
While the wave nature of a moving baseball is irrelevant to understanding its behavior, the wave nature of the electron is essential to understand its behavior in atoms. The crucial point, though, is that everything waves. An electron, a baseball, and you.
Quantum biology
De Broglie’s remarkable idea has been confirmed in countless experiments. In college physics classes we demonstrate how electrons passing through a crystal diffract like waves, with superpositions creating dark and bright spots due to destructive and constructive interference. Anton Zeilinger, who shared the physics Nobel prize this year, has championed diffracting ever-larger objects, from the soccer-ball-shaped C60 molecule (with 60 carbon atoms) to biological macromolecules.
The question is how life under such a diffraction experiment would behave at the quantum level. Quantum biology is a new frontier, one where the wave-particle duality plays a key role in the behavior of living beings. Can life survive quantum superposition? Can quantum physics tell us something about the nature of life?