Graphene is a Nobel Prize-winning “wonder material.” Graphyne might replace it

- Graphene is a “wonder material” made entirely of carbon atoms that has tremendous potential in the semiconductor industry.
- A related molecule, called graphyne, might be even better.
- Graphyne, however, is difficult to produce. Now, chemists have found a way to create it in bulk. Research can now get underway.
Since its synthesis in 2009, graphene has been dubbed a wonder material with applications in electronics, medicine, and energy, among other industries. On the other hand, graphyne — a similar material with subtle differences — has long evaded synthesis by chemists and chemical engineers. However, these tiny differences, researchers have hypothesized, would make graphyne a better choice for designing faster electronics.
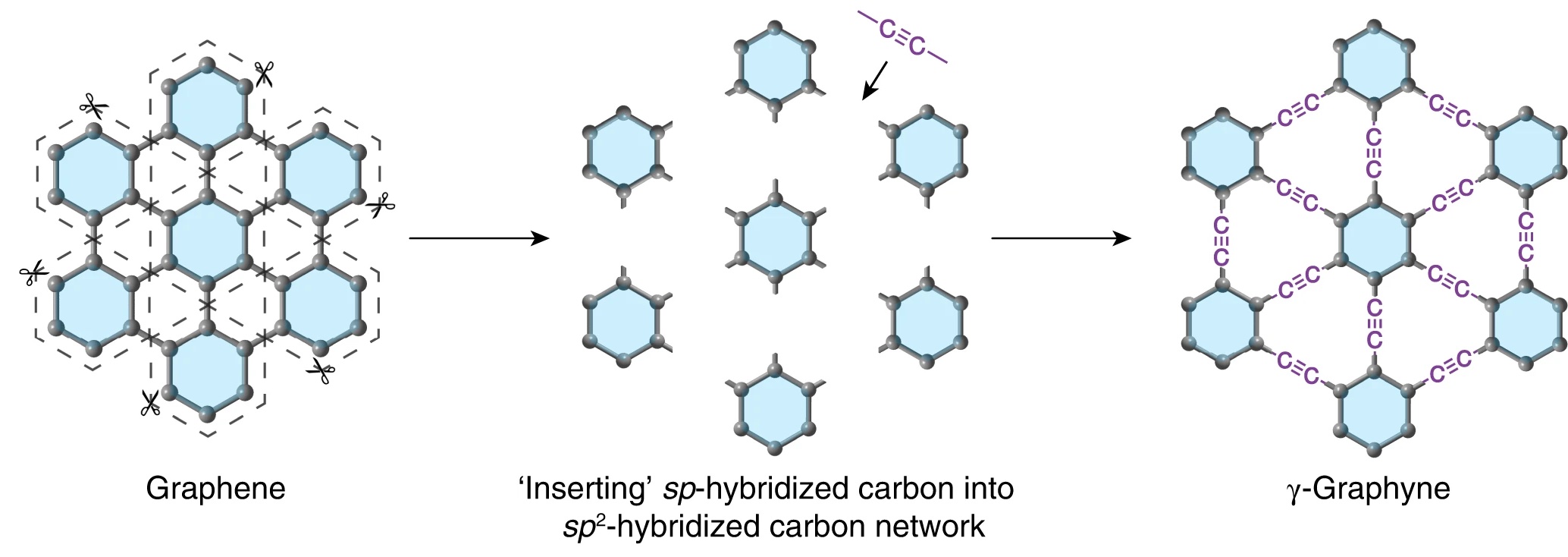
In research published in Nature Synthesis, scientists from the University of Colorado Boulder and Qingdao University of Science and Technology have reported the synthesis of bulk amounts of graphyne. Like graphene, it exists as a single layer of carbon atoms arranged in a symmetric lattice. Unlike graphene, whose atoms are tethered by single and double bonds, the carbon atoms in graphyne are bound to each other in single, double, and triple bonds.
Carbon: The amazing element
Some chemical elements exist in multiple physical forms known as allotropes. The atoms are arranged differently across allotropes, which provides them with different physical properties. The two best-known carbon allotropes are graphite and diamond. Both are pure carbon. However, in diamond, the carbon atoms are arranged in a compact lattice, resulting in its extreme hardness. On the contrary, the carbon atoms are arranged in loose layers in graphite, which explains its flakiness.
Of all elements, carbon has the richest diversity of allotropes, ranging from strong nano-sized tubes to 60-atom “buckyballs” to those that look like glass. There are two reasons why. First, carbon atoms can bind up to four different atoms at the same time. Second, carbon readily forms long chains and structures, even compared to other elements like silicon that can also bind four atoms simultaneously. (This is why extraterrestrial life is likely to be carbon-based, not silicon-based.) These carbon-carbon bonds are strong which, in turn, allows the element to form stable allotropes of various kinds.
Making graphyne
The focus of the current study was on γ-graphyne (“gamma” graphyne), the most stable isomer of graphyne. (Note: Allotropes and isomers are not the same. Allotropes do not necessarily have the same numbers of atoms, but isomers do. Isomers differ only by structure.)
Early approaches to synthesizing graphyne relied on irreversible chemical reactions. Consequently, any incorrect arrangements of carbon atoms persisted and caused the lattice to become unstable. In this study, the scientists used a reversible mechanism called alkyne metathesis, which redistributes chemical bonds in carbon chains, essentially allowing molecules to swap one portion of themselves for another on a different molecule.
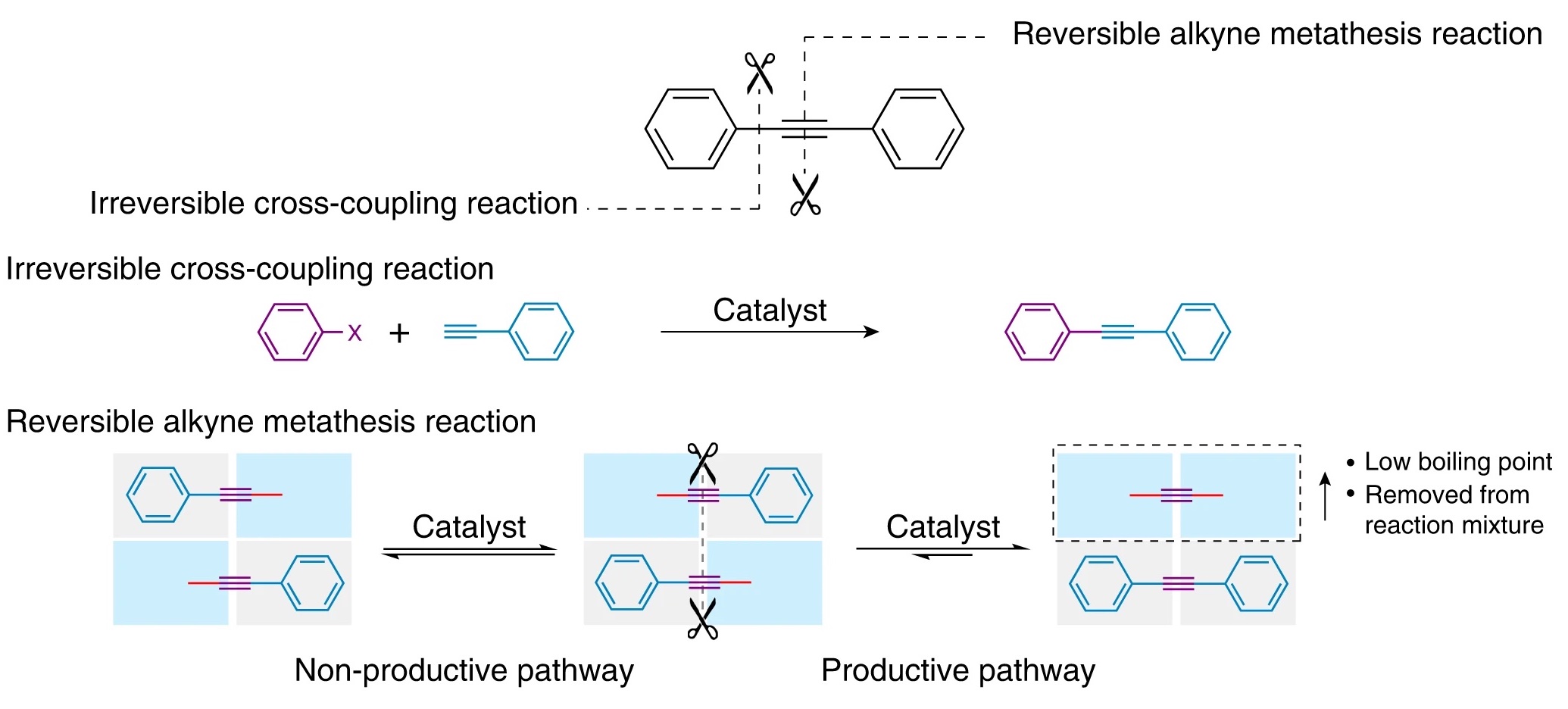
As shown above, the process uses metal catalysts to rearrange benzene rings (six-carbon molecules with alternating single and double bonds) in a periodic lattice connected by triple bonds.
Chemical reactions are tricky. Simply mixing together the ingredients you need does not guarantee a satisfactory outcome. The relative ratio of the products obtained differ depending on reaction conditions. Under “kinetic control,” the ratio of the products depends on the rates at which they are formed; under “thermodynamic control,” the more stable product is favored. To create graphyne — a large, stable lattice that is also error-free — the authors had to carefully balance these two methods of reaction control. To achieve this, the authors used two different benzene derivatives to construct graphyne. After several days, a dark black solid precipitated out of solution: γ-graphyne.
Will graphyne replace graphene?
Theorists have previously proposed a range of exciting mechanical, electronic, and optical properties for graphyne. This potentially has enormous implications for the semiconductor industry. Unlike graphene, its electronic properties are suggested to be direction-dependent due to its unique symmetry. It also has conducting electrons, eliminating the need for doping. Both of these qualities should make it a better semiconductor in comparison to graphene.
Now that chemists have a process to create meaningful amounts of it, research can really get underway.





