Which Elements Will Never Be Made By Our Sun?
The periodic table offers up so many possibilities, but some things are off-limits in our Solar System.
“There’s no god, it’s the elements that control this world and everything on it.” –Scott A. Butler
Our Sun is the greatest source of heat and light in the entire Solar System, fusing hydrogen into helium in a nuclear chain reaction in its core. Because an atomic nucleus of helium is 0.7% lighter than the four hydrogen nuclei that it’s created from, that act of nuclear fusion releases a tremendously efficient amount of energy. Over the course of its 4.5 billion year lifetime (so far), the Sun had lost about the mass of Saturn due to the amount of hydrogen that’s fused into helium, through Einstein’s E = mc^2, which is the root source of all the sunlight we receive here on Earth. The Sun has a lot more going on inside of it than just fusing hydrogen (the lightest element) into helium (the second lightest), though, and is capable of making so many more elements than that. But the periodic table has a whole slew of elements the Sun can never make.
We’re pretty fortunate that our Sun wasn’t among the very first stars in the Universe. Shortly after the Big Bang, the Universe was made exclusively of hydrogen and helium: 99.999999% of the Universe was composed of these two elements alone. Yet the first massive stars didn’t just fuse hydrogen into helium, but eventually fused helium into carbon, carbon into oxygen, oxygen into silicon and sulfur, and then silicon and sulfur into iron, nickel and cobalt. When the inner core reached a large enough concentration of those heavy elements, a catastrophic supernova occurred, creating a rapid burst of neutrons that were scattered into the other nuclei. Very quickly, the types of elements present in the Universe climbed up and up the periodic table, creating everything we’ve ever found in nature and many elements even heavier than that. Even the very first core-collapse supernovae created elements that are beyond the limit of what we find on Earth: elements heavier than even uranium and plutonium.

But our Sun won’t go supernova, and won’t ever make those elements. That rapid burst of neutrons that happens in supernova allows the creation of elements through the r-process, where elements rapidly absorb neutrons and climb the periodic table in great leaps and jumps. Instead, our Sun will burn through the hydrogen in its core, and then will contract and heat up until it can begin fusing helium in its core. This phase of life — where our Sun will become a red giant star — is something that happens to all stars that are at least 40% as massive as our own.

Reaching the right temperatures and densities, simultaneously, for helium fusion, is what separates red dwarfs (which can’t get there) from all other stars (which can). Three helium atoms fuse together into carbon, and then through another hydrogen-fusion pathway — the CNO cycle — we can create nitrogen and oxygen, while we can continue to add helium to various nuclei to climb up the periodic table. Carbon and helium make oxygen; carbon and oxygen make neon; carbon and neon make magnesium. But two very particular reactions take place that will create the vast majority of elements we know:
- carbon-13 will fuse with helium-4, creating oxygen-16 and a free neutron, and
- neon-22 will fuse with helium-4, creating magnesium-25 and a free neutron.

Free neutrons aren’t created in great abundance, just in relatively scarce numbers, since such a small percentage of these atoms actually are carbon-13 or neon-22 at any given time. But these free neutrons can only stick around for about 15 minutes, on average, until they decay away.
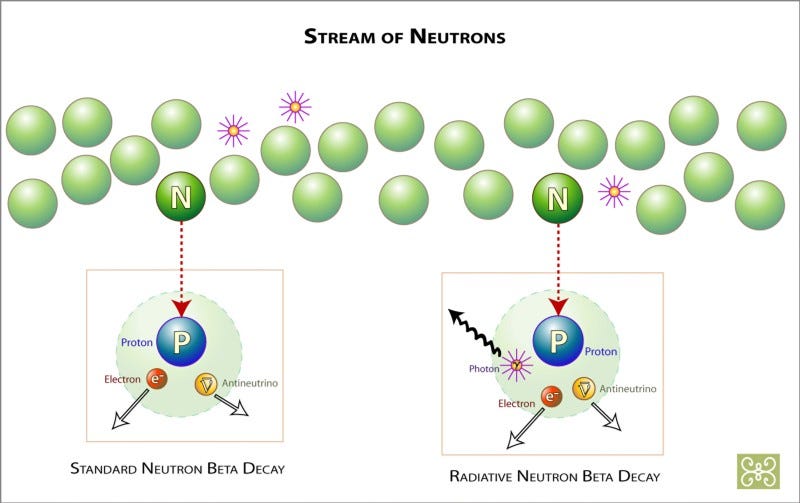
Fortunately, the interior of the Sun is dense enough that 15 minutes is more than enough time for this free neutron to run into another atomic nucleus, and when it does, it inevitably gets absorbed, creating a nucleus that’s one atomic mass unit heavier than before the neutron was absorbed. There are a few nuclei this won’t work for: you can’t create a mass-5 nucleus (out of helium-4, for instance) or a mass-8 nucleus (out of lithium-7, for examples), since they’re all inherently too unstable. But everything else will either be stable on timescales of at least tens of thousands of years, or it will decay by emitting an electron (through β-decay), which causes it to move one element up the periodic table.
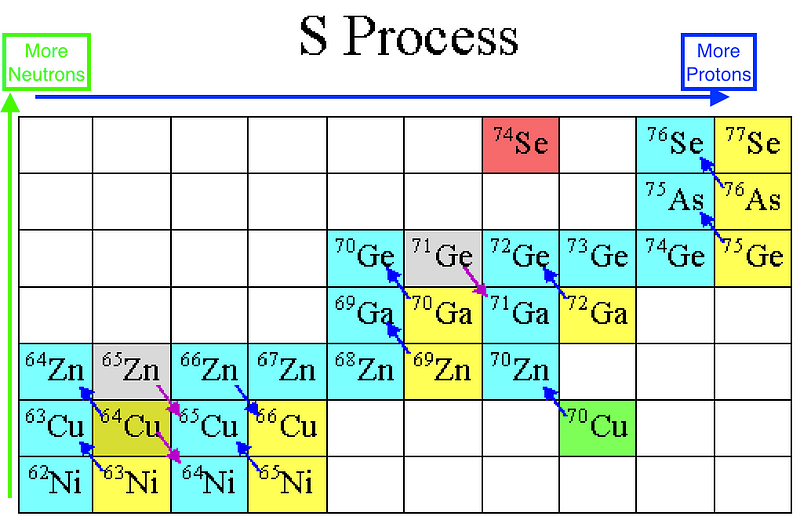
During any star’s red giant, helium-burning phase, this enabled you to build all the elements between carbon and iron through this process of slow neutron capture, and heavy elements from iron all the way up through lead through that very same process. This process, known as the s-process (because neutrons are produced-and-captured slowly), runs into a problem when it tries to build elements heavier than lead. The most common isotope of lead is Pb-208, with 82 protons and 126 neutrons. If you add a neutron to it, it beta decays to become bismuth-209, which can then capture a neutron and β-decay again to become polonium-210. But unlike the other isotopes, which live for years, Po-210 only lives for days before emitting an alpha particle — or a helium-4 nucleus — and returning back to lead in the form of Pb-206.
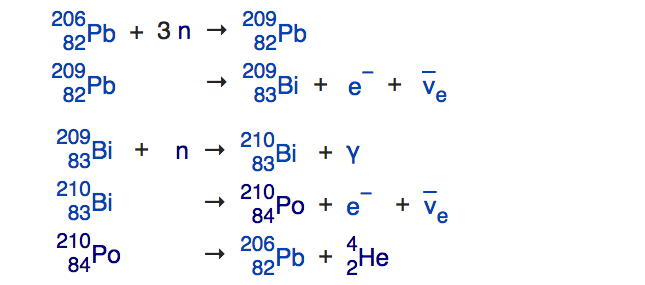
This leads to a cycle: lead captures 3 neutrons, becomes bismuth, which captures one more and becomes polonium, which then decays back to lead. In our Sun and in all stars that won’t go supernova, that’s the end of the line. Combine that with the fact that there’s no good pathway to get the elements between helium and carbon (lithium, beryllium and boron are produced from cosmic rays, not inside of stars), and you’ll find that the Sun can make a total of 80 different elements: helium and then everything from carbon through polonium, but nothing heavier. For that, you need a supernova or a neutron star collision.

But think about that: of all the naturally occurring elements here on Earth, the Sun makes about 90% of them, all from a tiny, non-descript star of no particular cosmic significance. The ingredients for life are literally that easy to come by.
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!





