What Are The Fifth And Sixth States Of Matter?
Solid, liquid, and gas are the three everyone learns. Plasma is the fourth. But there are two more, and they’re fascinating.
How many states of matter are there? When you were young, you probably learned about the three that are most common to our experience: solid, liquid, and gas. All of these occur with regularity here on Earth’s surface: rocks and ices are solids, water and many oils are liquids, while the atmosphere that we breathe is a gas. These three common states of matter are all based on neutral atoms, however; restrictions that the Universe is not bound by.
If you bombard any atom with enough energy, you’ll kick the electrons off of it, creating an ionized plasma: the fourth state of matter. But there are two additional states of matter that exist: Bose-Einstein Condensates and Fermionic Condensates, the fifth and sixth states of matter. At present, they’re only achievable under extreme laboratory conditions, but they might play an important role in the Universe itself. Here’s why.
Here on Earth, everything is made up of atoms. Some atoms bind together to form molecules; other atoms exist as standalone entities. Regardless of the number of atoms in any particular chemical compound — water, oxygen, methane, helium, etc. — the combination of temperature and pressure conditions determines whether it’s a solid, liquid, or gas.
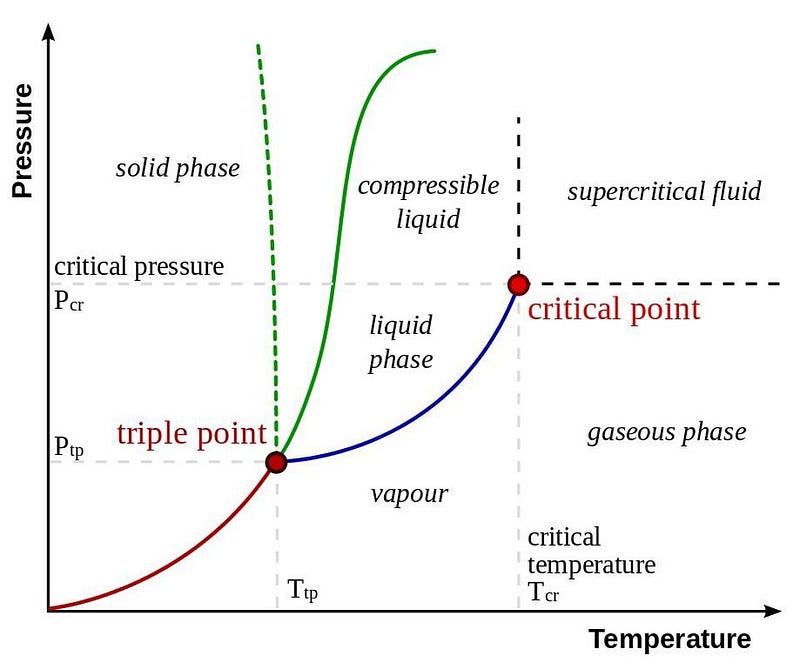
Water, most famously, freezes at low temperatures and modest pressures, becomes liquid at either higher pressures and/or higher temperatures, and becomes a gas at still higher temperatures or very low pressures. There’s a critical temperature, however, above about 374 °C (705 °F), at which this distinction breaks down. At low pressures, you still get a gas; at higher pressures, you get a supercritical fluid with properties of both gas and liquid. Go to higher temperatures still, and you’ll begin ionizing your molecules, creating a plasma: that fourth state of matter.
Although that’s where most discussions of states of matter end, that’s hardly the end of the scientific story. In truth, that’s just the end of the atomic part of the story. For the rest, we need to venture into the subatomic world: the world of particles smaller than the atom. We’ve already met one of them: the electron, which is one of the fundamental particles of the Standard Model.
Electrons are the negatively charged particles in atoms that orbit the atomic nucleus, the same particles that get kicked off at high energies to form an ionized plasma. The atomic nucleus, meanwhile, is made up of protons and neutrons, which in turn are made of three quarks apiece. Inside protons and neutrons, gluons, as well as quark-antiquark pairs, are constantly created, destroyed, emitted and absorbed within each of these composite particles. It’s a messy subatomic world inside every proton and neutron.
Here’s the key point that will lead us to the fifth and sixth states of matter: every particle in the Universe, no matter whether it’s a fundamental or a composite particle, falls into one of two categories.
- Fermion. This is a particle that, when we measure its spin (or intrinsic angular momentum), we always get values that are quantized in half-integer values of Planck’s constant: ±1/2, ±3/2, ±5/2, etc.
- Boson. This is a particle that, when we measure its spin, we always get values that are quantized in integer values of Planck’s constant: 0, ±1, ±2, etc.
That’s it. In all the known Universe, there are no particles — fundamental or composite — that fall into any other category. Everything that we’ve ever measured behaves either as a fermion or a boson.
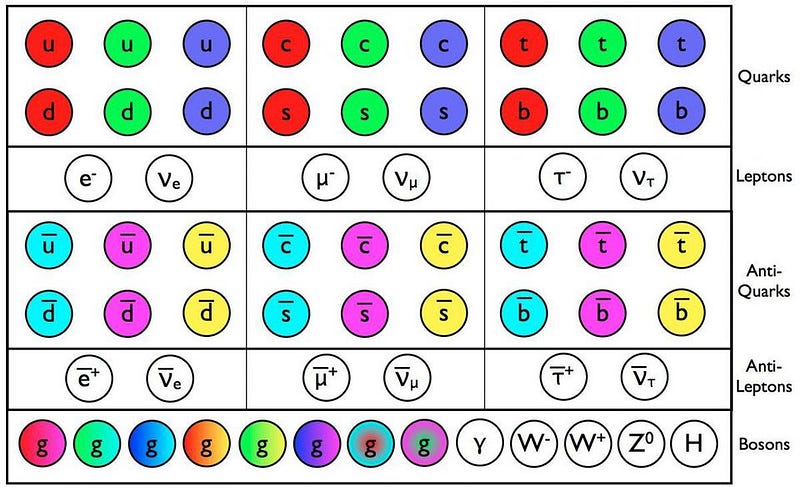
Electrons, being fundamental particles with spins of ±½, are obviously fermions. Protons and neutrons, each of which are made up of three quarks apiece, also have spins that can only be ±½, as the spin of one quark will always oppose the spin of the other two. However, if you bind a proton and a neutron together, you create a composite particle known as a deuteron: the atomic nucleus of a heavy isotope of hydrogen known as deuterium.
A deuteron, which is a fermion bound together with another fermion, always behaves as a boson. (Why? Because ±½ + ±½ can only equal -1, 0, or +1: the spin values for a boson.) Whether we’re dealing with fundamental or composite particles, fermions and bosons exhibit a key difference from one another. Yes, their spins are different, but that difference leads to an amazing consequence: fermions obey the Pauli Exclusion Principle; bosons do not.

The Pauli Exclusion Principle is one of the key cornerstones that was discovered in the early days of quantum mechanics. It states that no two fermions can occupy the exact same quantum state as one another.
This comes into play when we start putting electrons onto a fully ionized atomic nucleus. The first electron will sink down to the lowest-energy configuration possible: the ground state. If you add a second electron, it will also try to get down to the ground state, but will find that it’s already occupied. To minimize the energy of its configuration, it drops into the same state, but needs to have its spin reversed: +½ if the first electron was -½; -½ if the first was +½. Any further electrons need to go into a progressively higher and higher energy state; no two electrons can have the same exact quantum configuration in the same physical system.
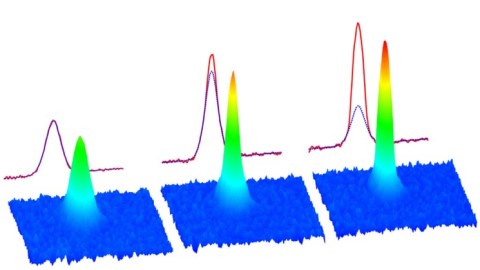
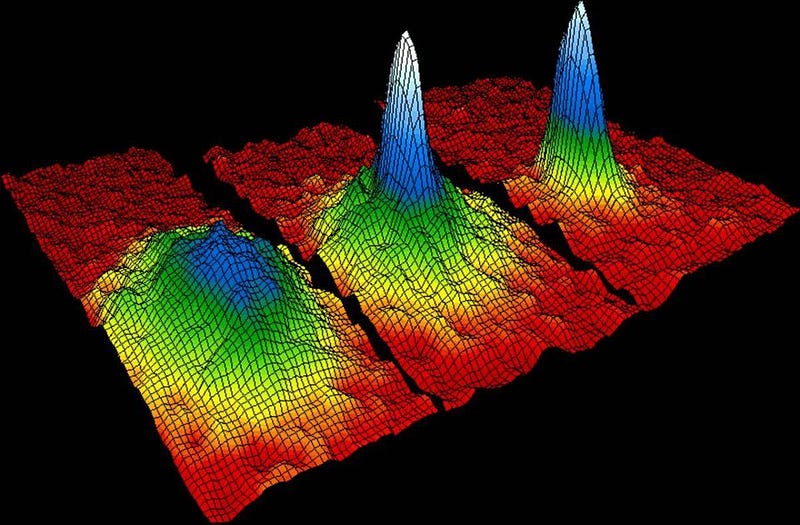
But this is not true for bosons. You can place as many bosons in the ground-state configuration as you like, with no restrictions. If you create the right physical conditions — such as cooling a system of bosons and confining them to the same physical location — there is no limit to the number of bosons that you can fit into that lowest-energy state. When you reach this configuration, of many bosons all in the same, lowest-energy quantum state, you’ve achieved the fifth state of matter: a Bose-Einstein condensate.
Helium, an atom made of two protons, two neutrons, and four electrons, is a stable atom made of an even number of fermions, and therefore behaves as a boson. At low enough temperatures, it becomes a superfluid: a fluid with zero viscosity and no friction between itself or any container that it interacts with. These properties are a consequence of Bose-Einstein condensation. While helium was the first boson to achieve this fifth state of matter, it has since been reproduced for gases, molecules, quasi-particles and even photons. It remains an active area of research today.
Fermions, on the other hand, cannot all be in the same quantum state. White dwarf stars and neutron stars don’t collapse because of the Pauli Exclusion Principle; electrons in adjacent atoms (in white dwarfs) or neutrons that border one another (in neutron stars) cannot fully collapse under their own gravity, because of the quantum pressure provided by the Pauli Exclusion Principle. The same principle that’s responsible for atomic structure keeps these dense configurations of matter from collapsing down to black holes; two fermions cannot occupy the same quantum state.
So how, then, can you achieve the sixth state of matter: a Fermionic condensate? Believe it or not, the story of Fermionic condensates goes all the way back to the 1950s, with an incredible discovery by the Nobel-winning physicist Leon Cooper. The term you’ll want to remember is named after him: Cooper pairs.
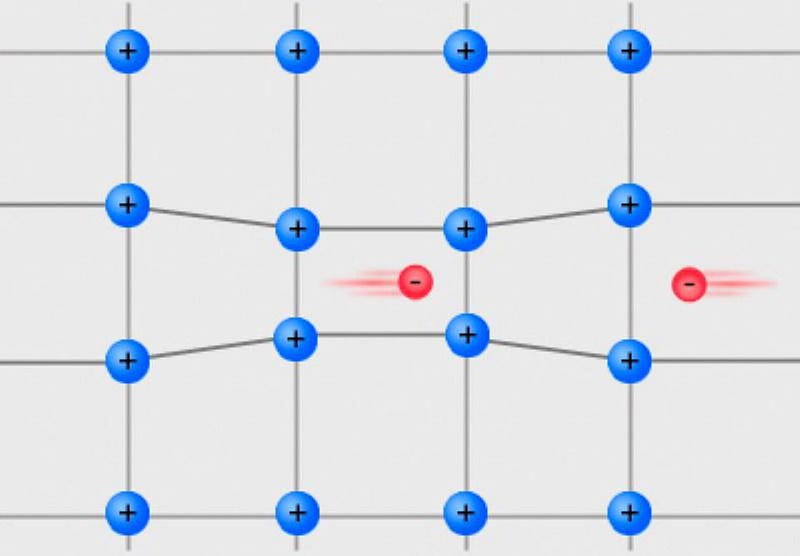
At low temperatures, every particle tends towards its lowest-energy, ground-state configuration. If you take a conducting metal and lower the temperature sufficiently, two electrons of opposite spins will pair together; this tiny attraction will cause electrons to pair up as a less energetic, more stable configuration than to have all your electrons moving individually.
Fermionic condensates require lower temperatures than Bose-Einstein condensates do, but they also behave as a superfluid. In 1971, helium-3 (with one fewer neutron than standard helium) was shown to become a superfluid at temperatures below 2.5 millikelvin, the first demonstration of a superfluid involving only fermions. In 2003, physicist Deborah Jin’s laboratory created the first atomic-based Fermionic condensate, leveraging a strong magnetic field along with ultra-cold temperatures to coax the atoms into this sought-after state.
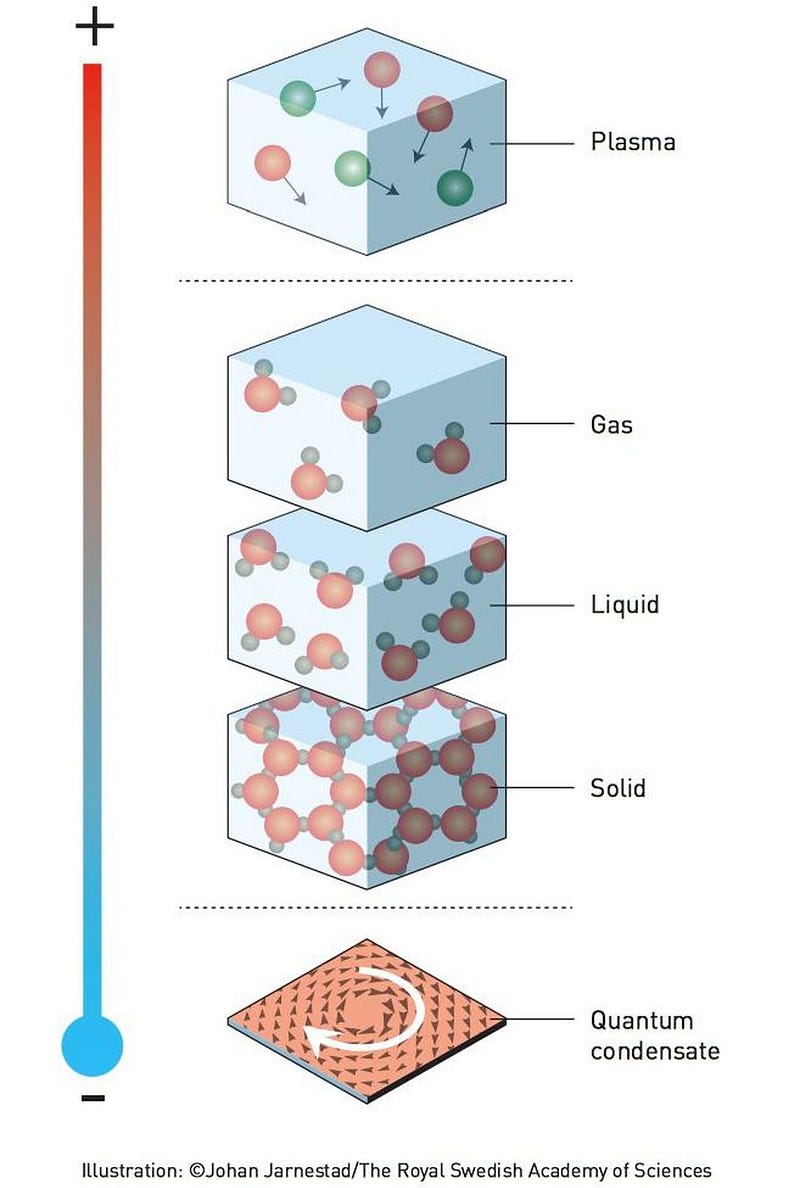
In addition to the three standard states of matter — solid, liquid, and gas — there’s a higher-energy state of an ionized plasma, arising wherever atoms and molecules have too few electrons to be electrically neutral. However, at ultra-low temperatures, the two fundamental classes of particles, bosons and fermions, can each condense together in their own particular fashion, creating Bose-Einstein or Fermionic condensates, respectively: the fifth and sixth states of matter.
In order to create a Fermionic condensate out of matter, however, you have to achieve extraordinary conditions: temperatures below 50 nanokelvin with an applied time-varying magnetic field. However, in the vast abyss of space, it’s eminently possible that neutrinos (made of fermions) or dark matter (which could be fermions or bosons) clump together to form their own condensates. The key to unlocking one of the greatest mysteries of the Universe might lie in the rarest and most extreme of all the known states of matter.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.





