Throwback Thursday: How stable is matter?
Will we stick around for eternity? Or someday decay away?
“I trust in nature for the stable laws of beauty and utility. Spring shall plant and autumn garner to the end of time.” –Robert Browning
Like everything else that we’ve ever directly observed in the Universe — galaxies, stars, and planets — human beings are made out of particles found in the Standard Model of elementary particles. And in particular, our bodies (along with galaxies, stars and planets) are made up of specific combinations of only a few of those particles: we’re made of atoms.
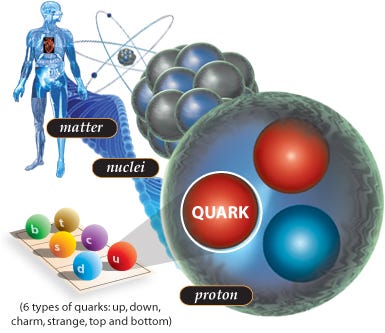
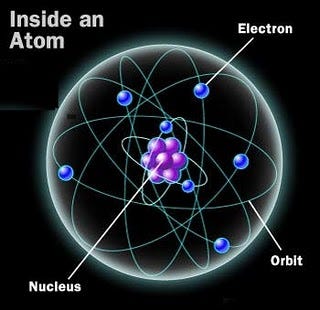
Atoms, in particular, are a special form of matter. They consist of bound states of electrons orbiting around an atomic nucleus. While the strength of the electromagnetic force and the interaction between the (negatively charged) electrons and the (positively charged) nucleus determines the size of the atom, the mass of the atom itself — at least, 99.94% of it — is determined solely by the atomic nucleus.
And if you go inside the nucleus of each atom, into the heart of them, you’ll find that these nuclei are combinations of just two types of nucleon: the proton and the neutron. Bound together in hundreds of different combinations, protons and neutrons not only determine what type of element your atom is, they also determine whether or not your atom is stable.
Inside the human body, there are, literally, more than 10^28 atoms that make you up.
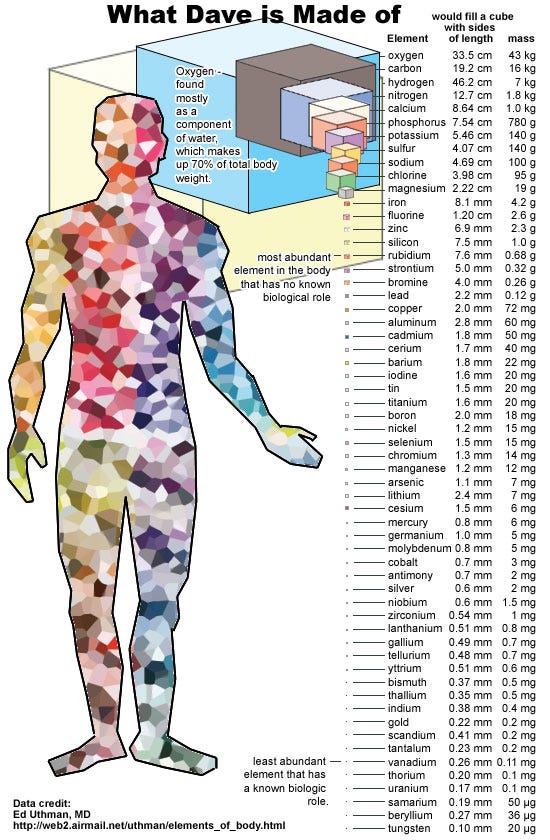
That’s right: more than 10,000,000,000,000,000,000,000,000,000 atoms in every single human body. Now, some of these atoms are well known to be radioactive, like bismuth, uranium, and thorium, but even these elements always conserve the total number of nucleons; they either transform a neutron into a proton and emit other particles in the process, or they split apart, emitting a helium nucleus from the main, parent nucleus.
Even a free neutron — unstable though it is — decays into a proton (and some other stuff), conserving the total number of nucleons.
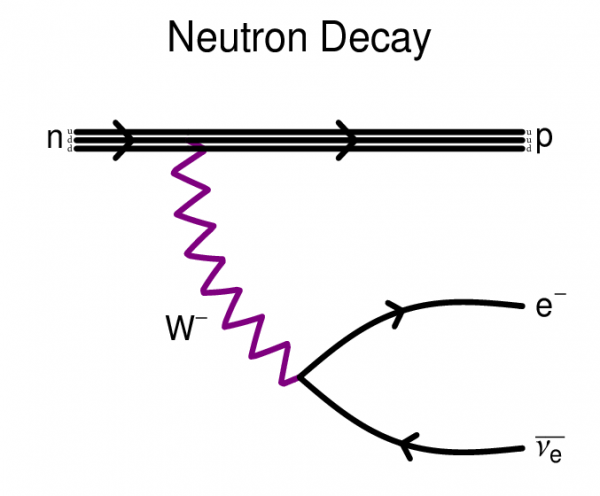
But what about the protons, you might ask? More than 10²⁷ of the atoms in every human are simple hydrogen atoms, with just a single proton for a nucleus.
Is it possible that these protons themselves are unstable? According to many ideas in physics (such as Grand Unified Theories), the proton itself could conceivably decay!
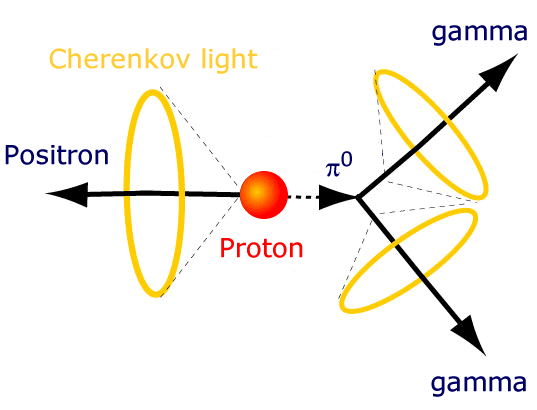
But if it does decay, it must be very long lived. Unlike a neutron, which decays after 15 minutes or so, the proton must have a lifetime that’s incredibly long.
We can figure this out just by using our bodies! With (to be a little more precise) 4 x 10²⁷ plain old protons inside you — from the nuclei of your hydrogen atoms — you couldn’t have too many of them decaying, or you yourself would release too much energy!
How’s that?

The same conversion of matter into energy that drives the Sun and atomic bombs — that same E = mc^2 — could also result from something as seemingly benign as a proton being inherently unstable. Whenever a particle of matter radioactively decays, that radioactive energy comes from the mass difference of the products and reactants, and from E = mc^2.
Well, you know what? Humans do emit energy that’s been internally generated, like all warm blooded creatures.

It isn’t obvious in visible light, but if you look in infrared light, you get to see that humans, relative to their outside environments, are constantly radiating their heat away to the cooler air around them.

In order to keep yourself at the proper temperature, you need to expend energy to make up for what you’re constantly radiating away. For a full-grown human about the size of a typical adult male, doing the calculation shows that each of us outputs around 100 Watts of power: that’s 100 Joules of energy every second, just like an old-school incandescent light bulb.

Even if you were getting 100% of this energy from decaying protons, that limits the number of protons that could decay each second, within your body, to no more than about 600 billion.
But based on the tremendous number of protons in your body (that’s where the figure of 4 x 10²⁷ comes in), you can figure out that — on average — it takes at least hundreds of millions of years for a typical proton to decay. Now in reality, humans are like all other warm blooded creatures, and we don’t get our 100 Watts of power from decaying protons.

We get it from chemical energy, mostly from eating bunnies. No, just kidding; we get it from eating calorie-rich foods. It takes about 2,000 food calories a day just to keep an adult male’s body temperature normal. (In fact, one of the earliest symptoms of undernourishment is a drop in body temperature.)
But if we want to test as accurately as possible whether protons decay or not, you know how to do it. You get as many of them together as possible, build a giant detector around them, and look for the telltale signature of their decay.
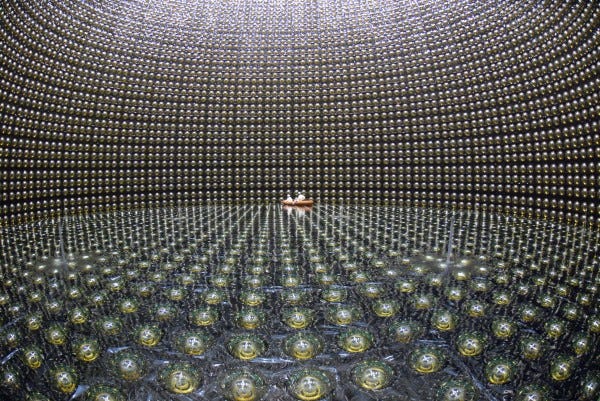
In Kamioka, Japan, they did just that. They built a tank with thousands of tonnes of water inside, and photon detectors all around the outside. If any of the protons decayed, the high-energy decay products would give off telltale light signals, allowing us to not only measure whether anything decayed, but how many of these atoms decayed.
You take a tank of 10³² protons, wait a year, and if none of them decay, you know that the proton has a half-life of at least 10³² years!
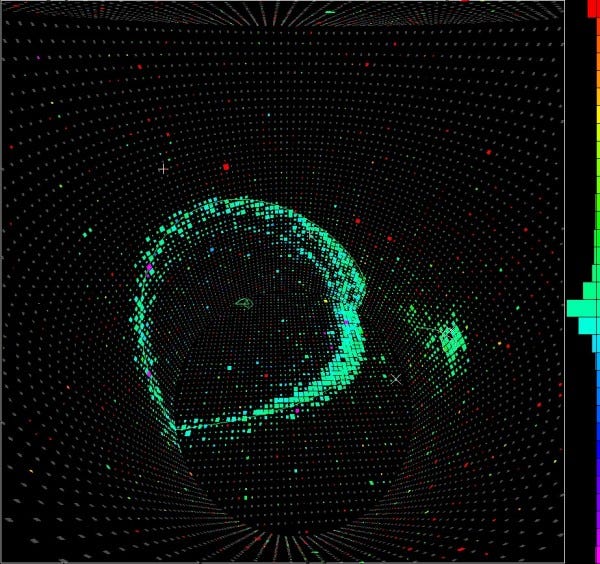
And while these setups of giant tanks have proven incredibly useful for detecting cosmic neutrinos, all the experiments ever done have given null results for proton decays. Overall, the best constraints we have tell us that the lifetime of a proton is at least 10³⁵ years, which is really not bad, considering that the Universe itself is only about 10¹⁰ years old!
The proton is so stable that it actually presents a problem for a significant number of Grand Unified Theories. In fact, just based on this constraint, we can say that, at most, there’s only a 0.001% chance of even one proton in your body decaying over your entire life, and that all the stars in the sky that will ever form will have burned out on timescales shorter than the average proton lifetime. But is it truly stable, or is it simply unstable on timescales longer than we’ve been able to measure?
That, at least, is still an open question.
Leave your comments on our forum, & support Starts With A Bang on Patreon!