The rarest light elements in the Universe
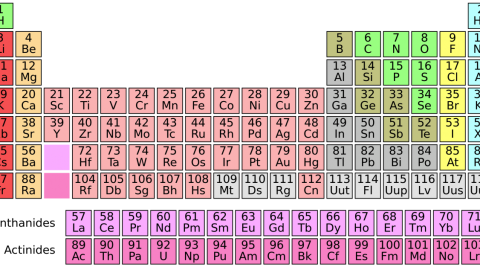
There’s a big gap between Helium and Carbon. Come find out why!
“And argon, krypton, neon, radon, xenon, zinc and rhodium,
And chlorine, cobalt, carbon, copper, tungsten, tin and sodium.
These are the only ones of which the news has come to Harvard,
And there may be many others, but they haven’t been discarvard.”
–Tom Lehrer
Immediately after the Big Bang, before the first stars in the Universe ever formed, the Universe consisted of hydrogen (element #1), helium (element #2) and pretty much nothing else. Despite originating from an incredibly hot, dense state, arbitrarily heavy elements weren’t created early on the same way they’re made today in stars. Despite being hot enough to make pretty much anything, the early Universe makes almost nothing for one simple reason: if it was hot-and-dense enough to fuse elements together in the very early stages, it was also hot enough to blast those composite elements apart again.
It’s only when the Universe has cooled enough that elements aren’t immediately split apart — a little more than three minutes in — that we can build our way up the periodic table.
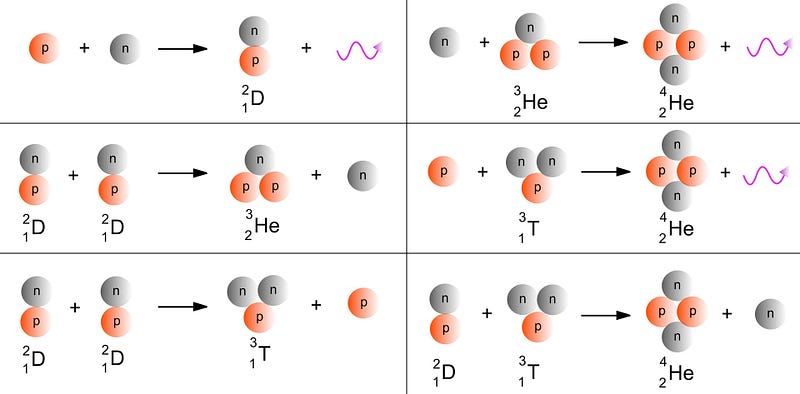
But even after just a few minutes, conditions are so low in energy that 99.999999% of the elements cap out at helium. And we don’t make anything new beyond that until we begin to form stars. Although the first stage of stellar burning always involves fusing hydrogen into helium in a star’s core, the stars that are massive enough (more than about 40% as massive as our Sun) will eventually build their way up the periodic table:
- When the star’s core runs out of hydrogen fuel, it contracts and heats up.
- When it reaches a temperature of about 100 million K, the helium ignites.
- With that ignition, helium burning commences, where three helium atoms fuse together to create carbon (element #6), releasing energy in the process.

This is the process at play in red giant stars, with more massive stars creating elements such as nitrogen, oxygen, neon, magnesium, silicon, sulphur and iron-cobalt-and-nickel. In addition, stellar burning also produces free neutrons, which can combine with the pre-existing elements to climb up the periodic table one element at a time, all the way up to elements like lead and bismuth (elements #82 and #83). And finally, the absolute most massive stars will die in a spectacular supernova explosion, leading to a runaway fusion reaction that — in principle — should produce everything that’s known in the periodic table and beyond, creating every element possible.
Every element possible, that is, except the three we skipped. You see, the Universe starts off with hydrogen and helium, all stars produce helium, and then stars over a certain mass threshold produce carbon, nitrogen, oxygen and lots of heavier elements. But carbon was already element #6; what about lithium, beryllium and boron (elements #3, #4 and #5)? When we look out at the Universe and the Solar System, and ask what the abundances of the elements are, we notice there’s a tremendous gap between helium and carbon, like those three elements are incredibly suppressed.
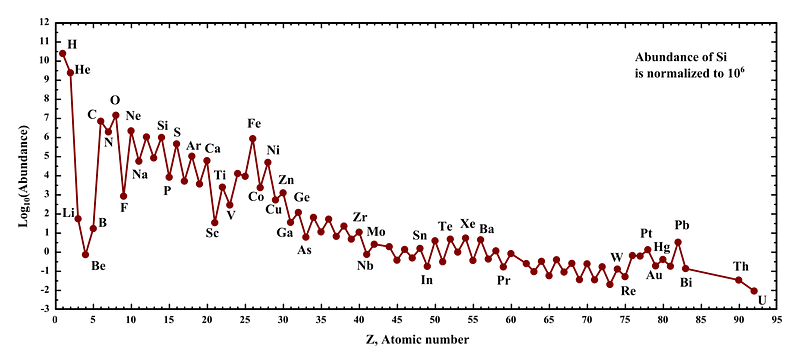
You can’t make those elements by fusing lighter ones together, since adding hydrogen to helium would create lithium-5, which is unstable, and adding two heliums together would create beryllium-8, which is unstable. (In fact, all nuclei with a mass of 5 or 8 are unstable.) You can’t make them from stellar reactions involving elements like carbon or above, since those only create heavier elements, not lighter ones. In fact, you can’t make the first of the heavier-than-helium elements in stars at all.
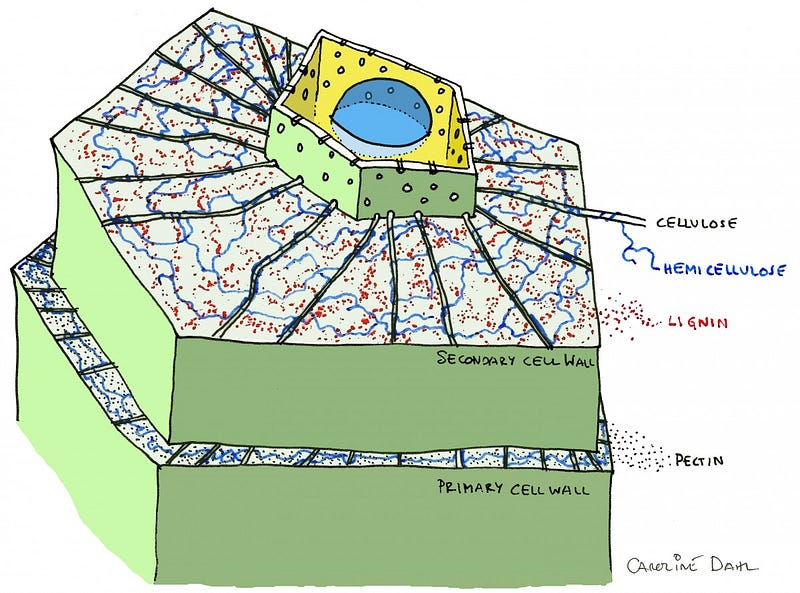
And yet, lithium, beryllium and boron not only exist, but boron in particular is vital for life-as-we-know-it on Earth. Without boron, there would be no such thing as a cell wall, and hence, no such thing as a plant. (For some of us the lithium batteries in our cellphones may be equally as indispensable!)
Yet plants exist, lithium, beryllium and boron exist, and so somehow, these elements must have been created. The keys, believe it or not, are the most energetic sources of particles in the Universe: black holes, neutron stars, supernovae and active galaxies. When these cosmic catastrophes ignite, become active or even explode, they don’t just emit particles. They emit the highest energy particles in the known Universe.
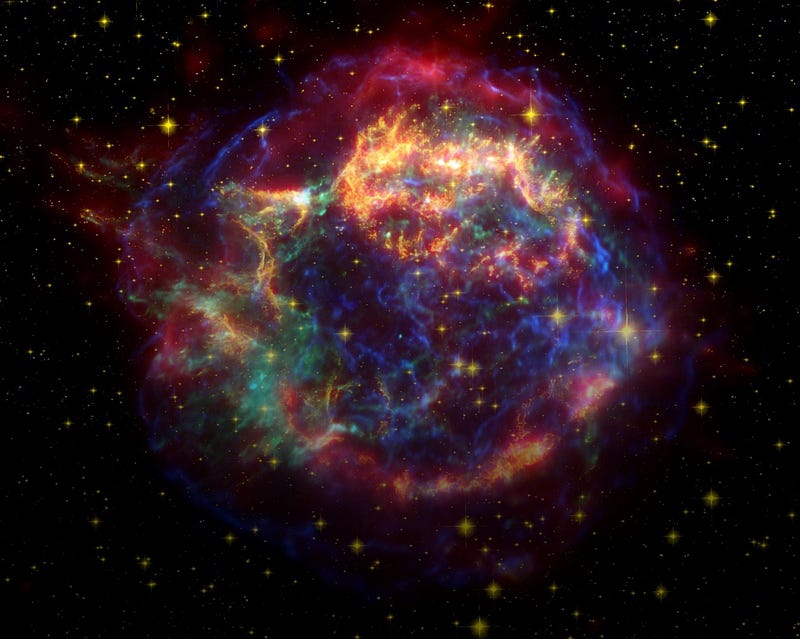
And when those energetic particles (known as cosmic rays) strike a heavier element — one created in a star — it can blast it apart, creating a cascade of lower-mass particles. This process, known as spallation, is how the lithium, beryllium and boron found on Earth was formed, and the only reason why these elements can be found at all on our planet. These three elements are by far the rarest of all the light elements, and this process is the only reason they’re around at all. The next time you see a plant, think not only of the evolutionary story that allowed it to be so, but the cosmic one, that enabled the elements essential to it to even exist. Without the most catastrophic, energetic events in the Universe, three of the lightest elements, lithium, beryllium and boron, simply would not be.
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!





