The Physics of Fireworks

After 4th of July this past Monday, here’s the science behind how they work!
“Celebrate the independence of your nation by blowing up a small part of it.” -The Simpsons
This past Monday marked the 240th anniversary of the United States’ independence from Britain, which is celebrated all across the country with tremendous displays of fireworks. From individual hobbyists to professional installations, fireworks all have the same physics behind them and the same four component stages: the launch, the fuse, the burst charge and the individual stars. Get it wrong, and you could be facing anything from a sub-optimal display to a dud to a dangerous fire hazard. But get it right, and the most spectacular shows of all are yours to behold.

You start with three simple ingredients: sulfur, charcoal, and a source of potassium nitrate. Charcoal, in this case, is not the briquettes you use on your grill, which often contain no actual charcoal, but is the carbon residue left behind by organic matter (like wood) once it has been charred (or pyrolyzed), having had all the water removed. Potassium nitrate is found in sources like bird droppings or bat guano. Take a mortar and pestle, mix them together, and what you’ll get is a fine, black powder. Gunpowder, in fact. All you need now is some oxygen — readily found in the potassium nitrate source (which means fireworks will even work on planets without oxygen in their atmosphere) — and a small source of heat. For the heat, so little is needed that even a lit match will do.
Add it all up, and you’ll get an explosion, accompanied by a deafening “boom” sound. But as much fun as that is, a simple explosion is hardly a firework! Sure, it’s part of a firework, but the real deal requires so much more. After all, if you’ve ever seen one, you know that the four major things that make a good firework are height, size, shape and color. The explosion itself is the catalyst for the latter three, but that’s just the starting point. As it turns out, physics has something to say about each one of these: the height, the size, the shape and the color of your firework!
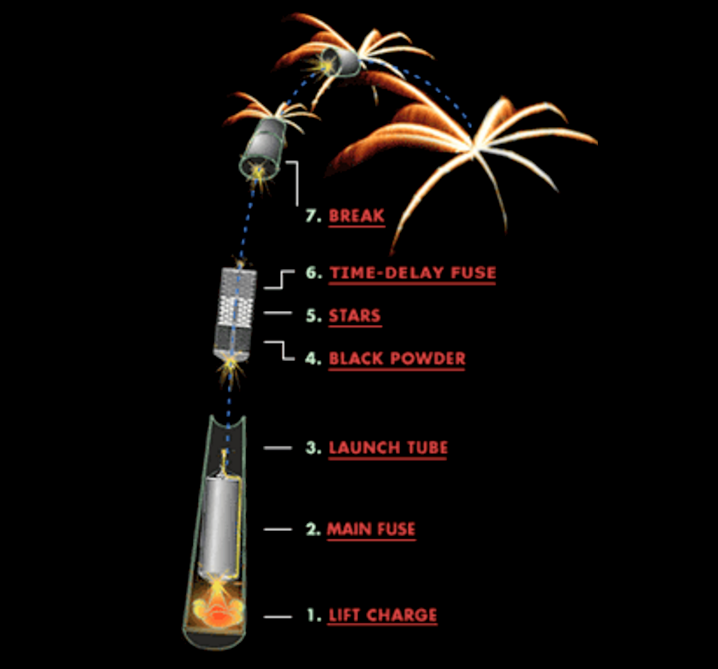
The height is the easiest one to explain, so let’s start there. The way you launch a firework is basically the same way you launch a cannonball out of a cannon! You put a “lift charge” in between the actual firework and the bottom of a strong, closed tube/pipe, and ignite it, propelling the firework up.
How high you want it to go is dependent only on the initial velocity of your firework, which is almost always larger for bigger fireworks. A small fireworks show might have 2″ (5 cm) to 6″ (15 cm) diameter shells being launched, which reach a height of anywhere from 200 to maybe 500 feet (60–150 m). But a very large fireworks show, like the one that takes place by the Statue of Liberty in New York City every year, uses fireworks with shells up to two or three feet in diameter (up to nearly a meter), and those fireworks often reach altitudes of well over 1,000 feet (300 meters).

Once the initial launch happens, the fuse on the actual firework itself — if all goes properly — should now be lit, with the ignition stage of the launch providing the spark to light the fuse. The fuse must be the proper length and burn duration so that it reaches the interior ignition stage at (or very close to) the apex of the firework’s height. For both aesthetic and safety reasons, you launch the larger fireworks to a higher altitude. (Aesthetics because more people can see them at higher altitudes; safety because a large, low-altitude explosion would be disastrous!)
The physics plays a central role with the size of your fireworks as well, because a larger firework not only requires a larger lift charge, but a larger explosive charge to propel the insides outward! The amount of the lift charge that you use has to be sufficient to launch the firework to the necessary altitudes described above, and that explains why larger fireworks get launched to higher altitudes.
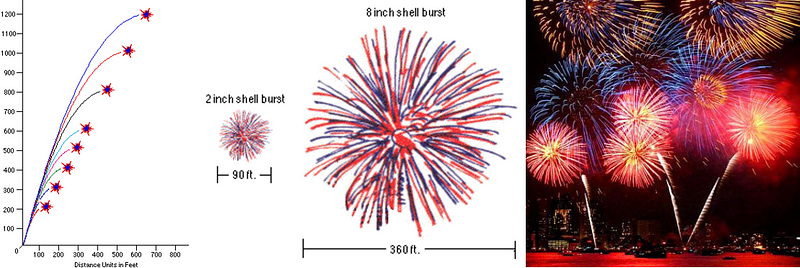
So long as they’re not “duds” (i.e., so long as the fuse ignites and burns properly), your firework will explode at or near the apex of its flight. The higher ones are usually larger, resulting in, well, aesthetically-pleasing (and again, safer) fireworks displays. But what determines their spectacular shapes that they come in? To find that out, we need to go inside the anatomy of a firework.
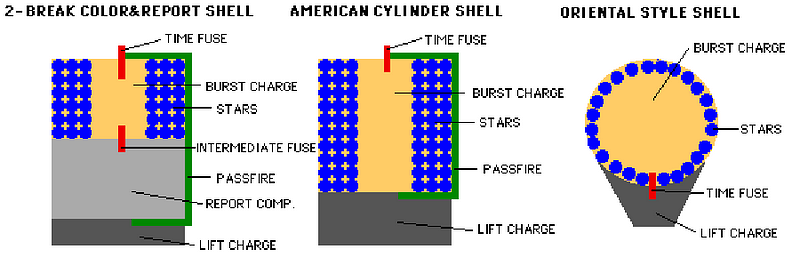
Fireworks come in many different styles, but the two important elements, once your firework has been launched into the air with its fuse lit, are the burst charge and the stars.
The burst charge can be as simple as more gunpowder, or it could be a more complicated (or even a multi-stage) explosive. The stars, on the other hand, are what actually go off in many directions, producing the beautiful display we’re all so accustomed to seeing. When the fuse burns down to the point where the flame reaches the burst charge, the charge ignites! This ignition, depending on how the firework is put together in the first place, will send the stars off into whatever pattern or direction it was designed for.

When the burst occurs, the temperatures get so hot that the individual stars that were contained inside ignite. This is where — for me — the most interesting part of the fireworks happens. In addition to whatever (optional) propulsion or fuel exists inside these stars, such as the ability to make them spin, rise, or thrust in a random direction, the stars are also the source of the light and color we find in our fireworks.

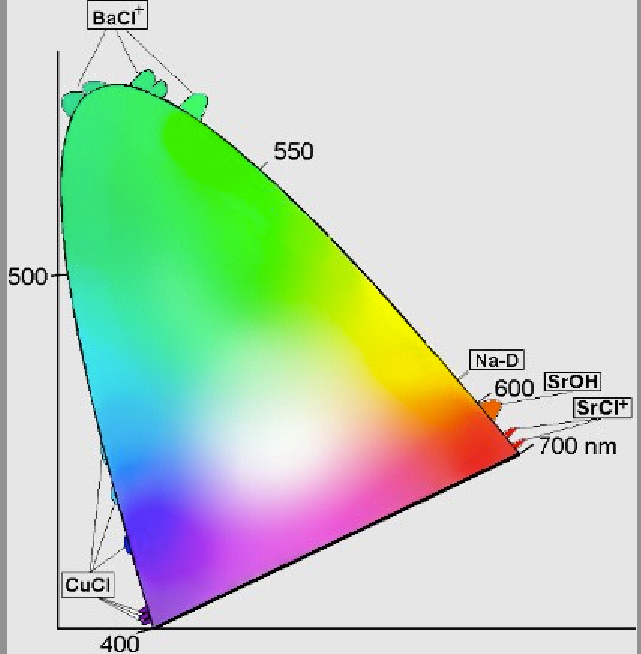
How are these “stars” responsible for color? Although there are some recent advances (covered in excellent detail by Janet Stemwedel back in 2007), the simplest explanation is that different elements and compounds have different characteristic emission lines. For example, if you take some sodium and heat it up, it emits a characteristic yellow glow, because of its two very narrow emission lines at 588 and 589 nanometers. (You’re probably familiar with them from sodium street lamps.)
We have a great variety of elements and compounds that emit a great variety of colors! Different compounds of Barium, Sodium, Copper and Strontium can produce colors covering a huge range of the visible spectrum, and the different compounds inserted in the fireworks’ stars are responsible for everything we see. Some notable ones are shown below in chromaticity space.
And that’s how fireworks work, from launch, up to the proper height, to their explosion, to the size, pattern, and color of the spectacular show they put on. Enjoy yours safely and to the fullest this fourth of July!
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!





