The Most Abused Principle in all of Science
How the mis-application of the Anthropic Principle has led factions of scientists away from the search for a natural, physical explanation of our Universe, and why that’s bad for everyone.
“There is a voice inside of you
That whispers all day long,
‘I feel this is right for me,
I know that this is wrong.’” -Shel Silverstein
As long-time readers of Starts With A Bang know, the end of the week brings with it a question of the week, and today’s comes all the way from Turkey and our reader Emre Oral, who asks:
Could you touch on the Anthropic principle and our finely-tuned Universe?
This is a very big question, so let’s start at the very beginning.

One of the first things you notice — and it’s self-evident if you think about it — is that the Universe is full of stuff. This in itself is a wondrous thing, because it didn’t need to be that way.
Our laws of nature, the physical laws governing the interactions of every particle in the Universe, including the forces that cause matter to bind, gravitationally interact, clump and cluster together, appear to be the same everywhere. We know how they affect all the known particles of the Universe, and they give us a framework for understanding how the Universe must have evolved to become what we see it as today.
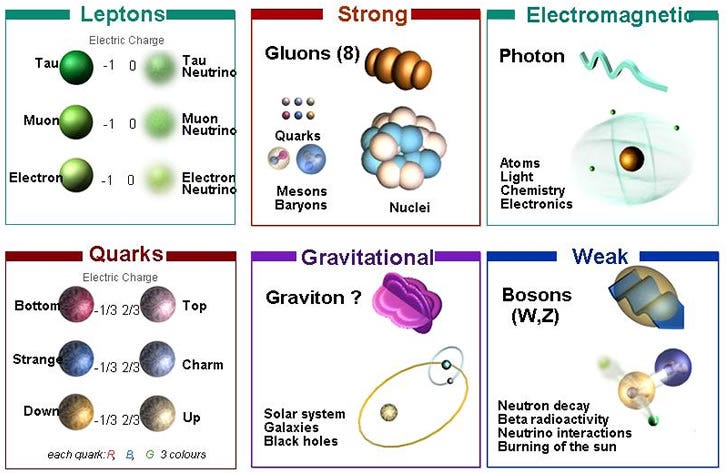
But knowing the laws of physics — knowing how all the different particles interact with one another — doesn’t answer all of our questions. Sure, it gets us very far: it tells us how a physical system behaves if you start it off with certain characteristics.
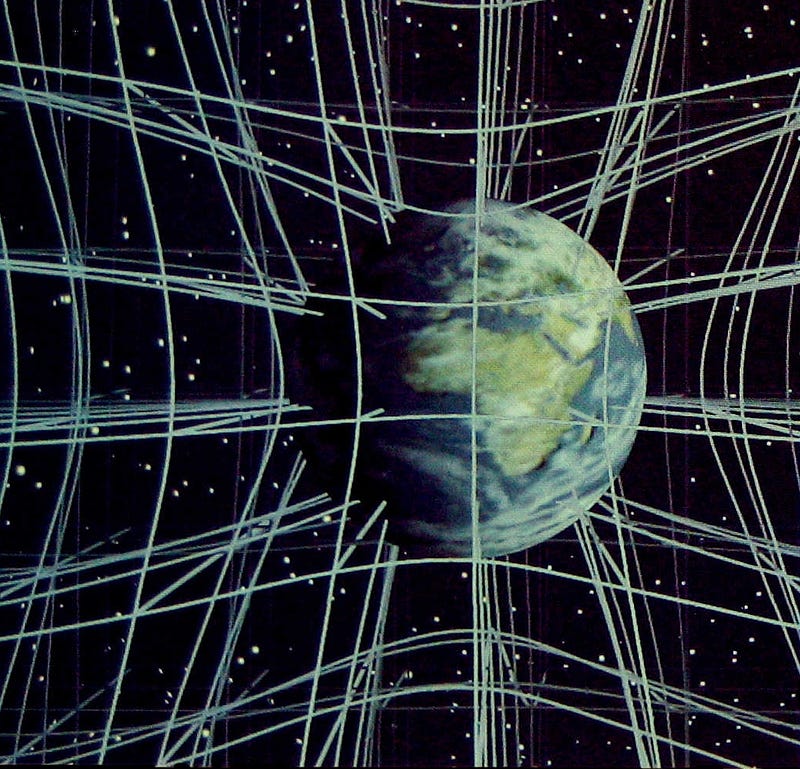
Spacetime expands or contracts, curving based on the matter and energy contained within it. Particles attract, repel or bind together based on the conditions under which they interact. Some systems will be stable; some will decay given enough time. The scientific process is very powerful, and does a remarkable job at telling us how these things happen.
But sometimes we need some guidance if we want to advance our knowledge and understanding of the Universe forward.
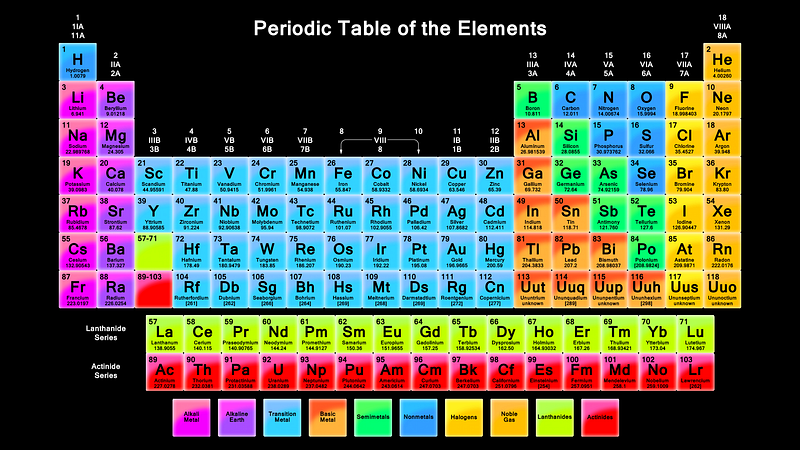
For example, we’ve long known that the Universe contains a huge variety of elements, or atoms with different numbers of protons in their respective nuclei. You, yourself, contain at least 59 different elements in your body in some capacity, yet for a long time we didn’t know how these elements came to be.
But we could always have been certain about one thing: we are here to observe the Universe.

This simple, self-evident fact actually carries a lot of weight. It tells us that our Universe does exist with such properties that an intelligent observer could possibly have evolved within it. That’s in contrast to properties that are incompatible with intelligent life, which cannot describe our Universe, on the grounds that no one would ever exist to observe it. That we are here to observe the Universe and the act of observing implies that the Universe is wired in such a way to admit our existence is the essence of the Anthropic Principle.
And on its own, just that realization can teach us a number of things.
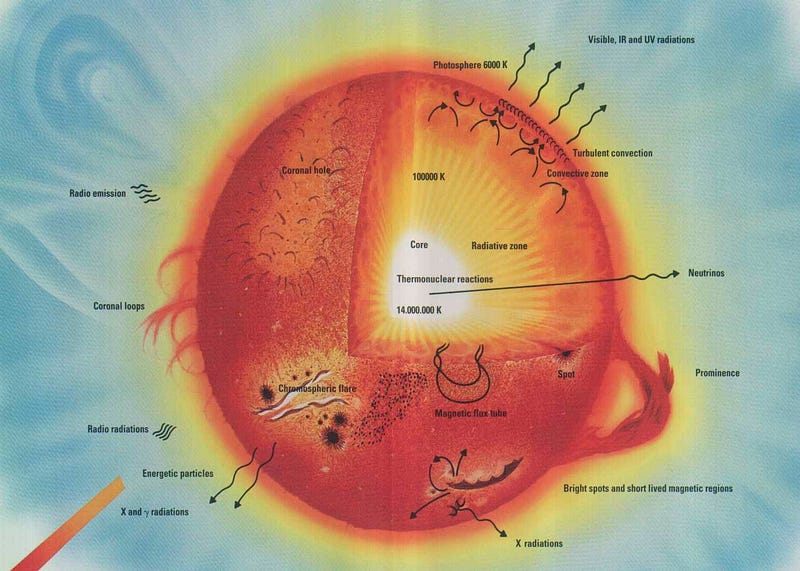
If our Universe is full of heavy elements, then there must have been some way to synthesize them! By the early 1950s, it was widely accepted that the stars were powered by nuclear fusion, and that our Sun was fusing hydrogen into helium over long periods of time. But those are the lightest two elements in the Universe! You couldn’t combine hydrogen (of mass 1) and helium (of mass 4) to move upwards, because there’s no such thing as a stable nucleus with a mass of 5, and you couldn’t combine two heliums, because beryllium-8 (of a nearly identical mass) is unstable, and decays back to two heliums on timescales of ~10^-16 sec.
But in 1952, Fred Hoyle used the anthropic principle to reason that there must be a process to create the heavier elements. He inferred that there must be a way to get a third helium in there — to interact with the highly unstable beryllium-8 — and fuse together to create carbon-12. The thing is, the masses didn’t match up! Carbon-12 has a much lower mass than beryllium-8 and helium-4 combined, so he made a breathtaking prediction: there must exist an excited state of carbon-12, one that nuclear physicists hadn’t yet discovered, that had precisely the mass of three helium-4 nuclei together.
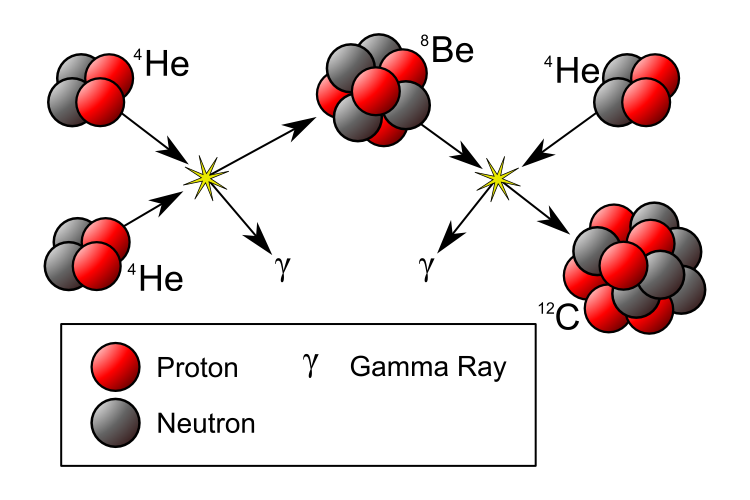
This was an incredibly bold prediction that flew in the face of known nuclear physics: such a state should have been discovered by then. But Hoyle told nuclear physicist Willie Fowler about it, and Fowler went to work searching for it. Five years later, the discovery of both the theoretical Hoyle State and the mechanism for forming it — the triple-alpha process — had been discovered and confirmed. And later that year, the two of them, along with Geoffrey and Margaret Burbidge, published a paper correctly explaining the origin of all the heavy elements in the Universe: the cores of giant stars that then go supernova, enriching the Universe!

The anthropic principle helps us to understand why the Universe’s properties must fall into a certain range of values.
Gravity couldn’t be too much stronger than it is, or the Universe would have been filled with black holes and nothing else. Dark energy (or the cosmological constant) couldn’t have been more than about 100 times larger than its observed value, or gravity wouldn’t have allowed us to form even a single star before the primeval atoms accelerated away from one another. There must be a fundamental matter-antimatter asymmetry in the Universe, because if there weren’t, there wouldn’t be enough “stuff” to create the Universe as we know it.
Although there are many variants of the Anthropic Principle, this is how I choose to state it:
The laws of nature must be such that the Universe can exist in a fashion consistent with what it is observed to be.
That’s pretty hard to argue with. And yet, by itself, it isn’t a scientific answer to any problems at all.

We know that there is a matter-antimatter asymmetry in the Universe, but knowing that we must have one in order to satisfy the anthropic constraints doesn’t tell us why the Universe has the matter (and not the antimatter) present within.
There is often an assumption that physicists make — and it is not necessarily a good assumption — that in principle, the laws and constants of nature could have taken on any number of arbitrary forms or values. If you accept that, then of course our Universe as we observe it must have laws and constants that are consistent with the existence of an intelligent observer.
But using that line of thinking, you’ll never understand how this happened.
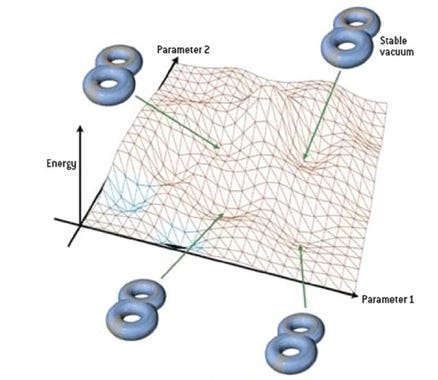
This line of non-scientific thinking rears its ugly head when people think about the cosmological constant (or dark energy) problem, asking why the Universe is finely-tuned to have the cosmological constant value it’s observed to have, some 10^120 orders of magnitude smaller than our naive prediction. The argument goes something like this:
Well maybe our naive calculations for the cosmological constant give us a number too big by a factor of 10^120, but The Landscape gives us 10^500 possible universes, and at least some of those are going to have the right value, and the others don’t matter because there’s nobody there.
That argument isn’t wrong so much as it is tantamount to giving up on physics, or the notion that the properties of our Universe are explicable and understandable in terms of physical laws and dynamics. There are plenty of other problems that have similar difficulties, such as the magnitude of the matter-antimatter asymmetry (off by 10 orders of magnitude of what we currently understand), the masses of the fundamental particles (19 orders of magnitude different from what we expected), and the relative weakness of the force of gravity (weaker than the others by over 30 orders of magnitude).
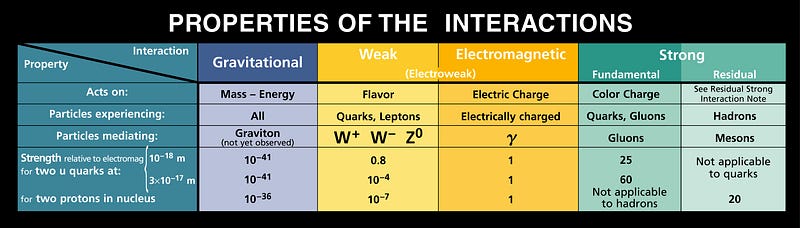
Any type of scientific reasoning is only useful when it tells you something you don’t already know, and we already know that we live in this Universe, and that it has the properties we observe. Saying “it must be so because we’re here” is both a logical fallacy (it could’ve been different, and we still might be here) and doesn’t teach us anything new. The Universe may be finely-tuned to a certain extent, but anthropics isn’t going to tell us why or how.
And that’s not enough. It’s not enough for me, and not enough for science. We wonder and we investigate so that we can figure out the answers, and that means understanding the dynamics. Anthropics can guide us — as they did Fred Hoyle more than 60 years ago — but it won’t give us a satisfactory answer, not on its own.
The search for knowledge continues.