Sorry, Methane And ‘Organics’ On Mars Are Not Evidence For Life

There’s a big difference between evidence and wishful thinking.
The story of how life arose on Earth is one of the great mysteries of modern science. We know, in many ways, how life evolved from a single-celled, relatively simple state billions of years ago to the diverse, complex, differentiated, and macroscopic organisms that populate our ecosystems and biosphere. We know that the early Solar System was rife with the ingredients for life, and that early Earth also had these ingredients, plus liquid water on its surface. Early on, in addition to Earth, young Mars likely had those same conditions as well.

But setting up the ingredients for life on a world along with conditions where it’s possible for life to arise is no guarantee of life. In fact, despite all our advances and achievements, we have not yet once created life from non-life in a scientific laboratory. Yesterday, NASA announced that its Curiosity rover had made two big discoveries:
- That there are ancient, hardy organic molecules found in 3-billion-year-old rocks there.
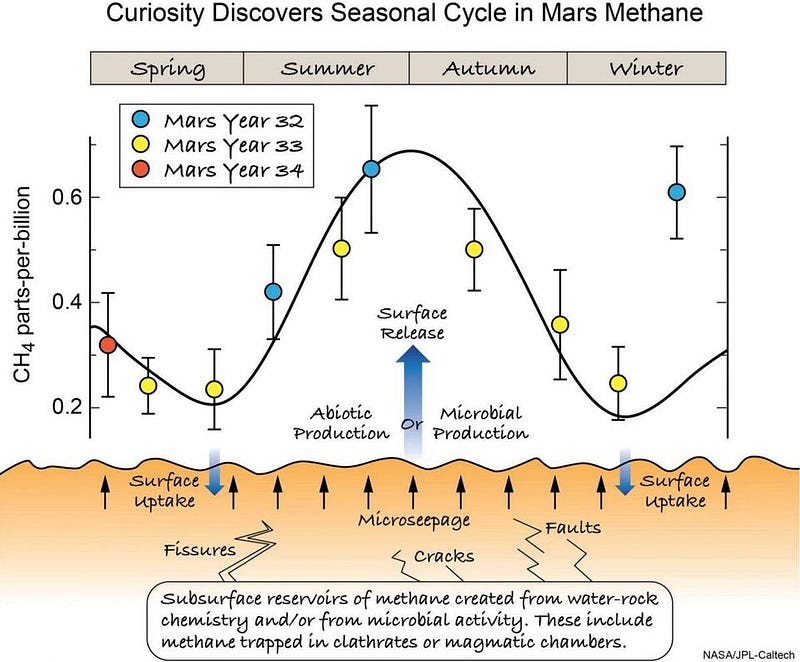
- That methane in the martian atmosphere varies with the seasons, repeatably, over the span of years.
These are no doubt very interesting finds. But do they imply life on Mars? Hardly.
First off, you have to realize that what you call an organic molecule and what a scientist calls an organic molecule are likely very different things. We tend to think of the word “organic” as meaning something that’s part of a living creature that came about through natural processes. Organic molecules might be fatty acids, proteins, sugars or starches, or even something as complex as DNA. These biologically interesting molecules are indeed organic, and essential to life as we know it. But to a scientist, the definition of an organic molecule is much more mundane: a molecule that contains at least one carbon atom.
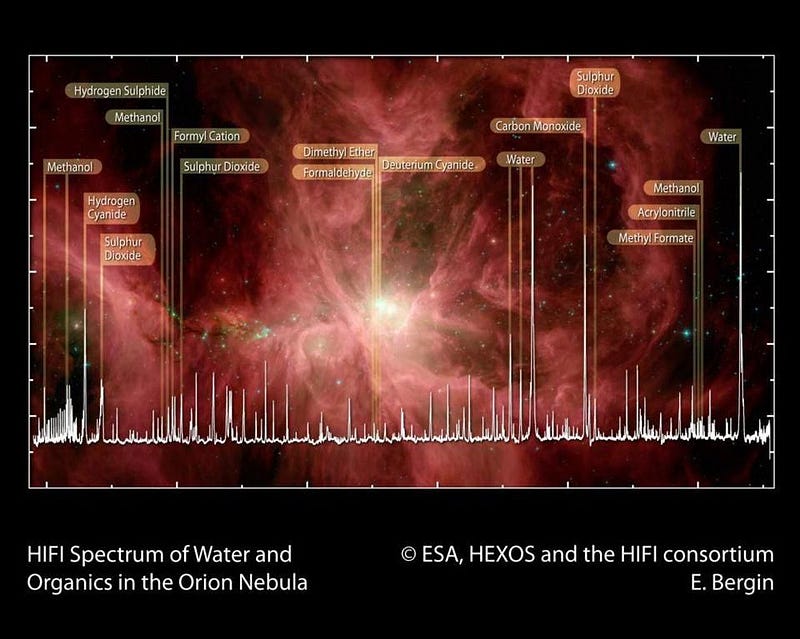
Since carbon is the fourth most abundant element in the entire Universe, behind only hydrogen, helium, and oxygen, it’s going to be pretty common wherever you look. Most stars have high carbon contents, so do all the rocky bodies we’ve found in our Solar System. Organic molecules can be as simple as carbon monoxide, and as harmful to life as cyanide. They exist in great abundance around young, massive stars, in interstellar gas clouds, in asteroids and meteorites, and on every world we’ve ever examined up close. Yes, including on Mars.

Mars is also interesting because it contains strong evidence for a past history of liquid water. There are layers of exposed sedimentary rock, flats full of hematite spheres, dried-up riverbeds with oxbow bends in them, and much more. Upon analysis, we’ve even found large amounts of ice frozen just beneath the surface, and evidence for presently flowing water along the surface, particularly down the slopes of crater walls.

Most of the Martian surface became dry and cold long ago, but for over a billion years, liquid water and life-giving temperatures were the norm. It’s speculative, but possible, that life arose there long ago, and that some forms of microbial life still survive. That’s been the great hope of many scientists working on Mars for generations. In the 1970s, the first landers (Viking 1 and 2) touched down on the Martian surface, and performed the famous Labeled Release (LR) experiment.
The Labeled Release (LR) experiment took a sample of Martian soil and applied a drop of nutrient solution to it, where all of the nutrients were tagged with radioactive carbon-14. Radioactive carbon-14 would then be metabolized into radioactive carbon dioxide, which should only be detected if life were present.

At least, that was the logic behind it. There was radioactive carbon dioxide detected, but there was a problem. You can also produce it inorganically, through purely chemical reactions. In 2008, the Mars Phoenix lander detected perchlorates in the soil, which could have been the cause of the first positive reading in the LR experiment. When heated, perchlorate can — in the presence of certain chemical compounds — produce chloromethane and dichloromethane, the exact compounds Viking 1 and 2 detected. The problem is that Martian soil, when exposed to intense UV radiation, could have created these compounds without the need for life at all.
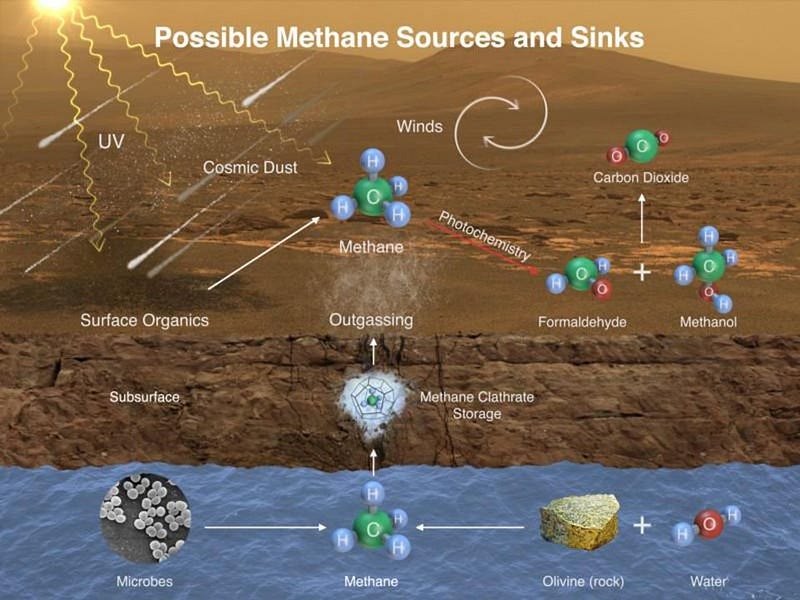
With Curiosity, we’ve now discovered that there’s methane being emitted from the Martian surface, and that the methane is seasonal in nature. This likely arises from a sub-surface mineral known as methane clathrate, which could arise in a number of different ways. Earth’s methane comes from mostly biological activities: mammals (like cows) and microbes. But you can create it simply by having water flow over and through certain sub-surface rocks, like olivine.
In science, if you want to explain something new, you always default to the simplest explanation. But what makes an explanation the simplest? It’s the one that requires the fewest number of new assumptions beyond the ones we already know must exist.

Gale crater, where Curiosity landed and takes data, was one a water-filled lake that has now dried up. The methane that it sees may have an ultimately organic origin, but there’s no reason to think this must be so. Inorganic processes may be what’s responsible here, and they account for the data in full, with no life processes required. Scientifically, it’s extremely interesting either way, as lead author of the new study, Jen Eigenbrode, recounts:
Whether it holds a record of ancient life, is the food for extant life, or has existed in the absence of life, organic matter in Martian materials holds chemical clues to planetary conditions and processes.

The good news is that even if this methane is geological in origin, that still teaches us something tremendous about Mars: it is geologically more active than we originally thought. The process that creates methane, geologically, is known as serpentization, which requires liquid water interacting with rocks in the presence of heat. That means some sort of interior activity must be occurring on the red planet. As Tanya Harrison writes:
To have liquid water interacting with rocks on Mars, that means you need a heat source. Until recently, scientists thought Mars’ core was solid and we’d seen no evidence for volcanic activity younger than about 100 million years ago. But the Mars Atmosphere and Volatile Evolution mission (MAVEN), which arrived in 2014, observed auroral activity at Mars. And this requires a magnetic field. So, perhaps Mars isn’t geologically dead on the inside after all! The InSight lander, on its way to the Red Planet right now, will help to answer this question.

In 2020, two next-generation rovers will launch: ESA’s ExoMars and NASA’s Mars 2020. Instead of indirect inferences and possibilities, we’ll actually be able to understand whether the origin of these molecules is geological or biological in nature. It’s important to keep an open mind and let science, rather than our hopes or fears, decide the answer. The evidence is building, and we’re finally gaining a more robust picture of how, exactly, Mars works.

It’s producing methane seasonally, contains loads of carbon-based compounds, and had a very watery past. But does that all add up to life, past or present? In 2018, the evidence doesn’t say “yes” just yet. But in just a few years, we just might have the answer. In a few years, for the first time, we might finally know if there’s life beyond Earth.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.





