What we wish we knew about the origin of life

- In some ways, Earth was a very fortunate planet, where life arose early on and has survived and thrived ever since.
- The precursor ingredients for life are everywhere all throughout the Universe, but, at least so far, Earth is the only known planet with extant life on it.
- In all the experiments we’ve ever conducted, despite all our attempts, we’ve never created life from non-life. Here’s where the greatest question marks are.
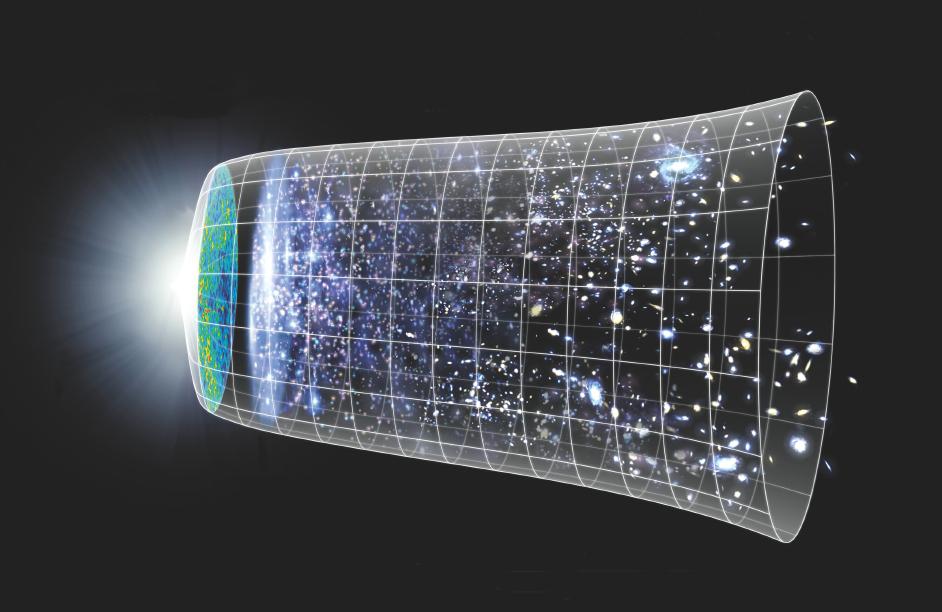
Out there in the Universe, there are hundreds of billions of stars in the Milky Way, each with their own set of planets, moons, and opportunities for life to arise. Beyond the Milky Way galaxy, there are approximately two trillion additional galaxies contained within the observable Universe, and a still larger, but hitherto undetermined amount beyond that. And yet, for all that we’ve learned and discovered about the cosmos that we inhabit, Earth remains the only planet we know of that presently has — or that ever has had — life on it.
It’s possible that life is not only out there, but abundantly so. The same raw ingredients that gave rise to life on Earth are found everywhere, and similar conditions are found all throughout the galaxy, with billions of Earth-like planets with similar orbits around similar stars. Additionally, there are planets both smaller and larger, moons around giant planets, and planets that orbit longer-lived stars than our own. It’s possible that life, including complex and even intelligent life, might be ubiquitous out there in the great cosmic ocean. But to make any sort of reasonable estimate, we have to solve one of the greatest mysteries in all of science: how life began on Earth. Here’s what we wish we knew.

Our Solar System, as far as we can tell, isn’t special or privileged in any way. The same laws of nature are in place here as in all other places and at all other times elsewhere in the Universe. The raw ingredients that formed our Solar System are found in equal, nearly-equal, or even greater abundances in stellar systems all over the galaxy and elsewhere, in other galaxies, throughout the cosmos. Not only is our Solar System, in many ways, completely unremarkable and non-unique, but we are among the most common configurations out there.
The raw ingredients needed for life — including both the heavy elements used in life processes and life-essential molecules like sugars, amino acids, and various organics — are found ubiquitously in space. They’re found:
- in outflows around hot, young stars known as Herbig-Haro stars,
- in the interstellar gas populating the galactic center,
- in protoplanetary disks found around newly-forming stellar systems,
- and even in the leftover relics from our own Solar System’s formation: comets and asteroids.
As far as we can tell, wherever there are stars rich in heavy elements, just like our own, there are also rocky planets and the precursor materials needed for life to arise.

In all life processes known to occur on Earth, only about 20 amino acids — all of which have a left-handed structure, or chirality, to them — play a role. And yet, when we examine even the raw ingredients found in the meteorites originating from our own Solar System, we’ve found that there are more than 80 total amino acids, and that they naturally occur in both left-handed and right-handed versions.
Despite all that we do know, including all that we’ve learned about life from our scientific investigations, we still cannot describe, in any sort of detail, how life first emerged, naturally, from non-life in our Universe.
We do know, at some point in the early history of Earth — likely during the planet’s first few hundred million years but possibly even earlier — life appeared on our surface. For at least 3.8 billion years, and possibly for as many as 4.5 billion years, it has survived, reproduced, and thrived, eventually leading to the enormous variety of complex and differentiated species that populate our planet today. But just what that first example of life was, including whether its descendants still exist today, remains a mystery.
The earliest environment of planet Earth was a very different place compared to what it is today, though there are some similarities. Shortly after the formation of the Moon, there were likely both continents and salty, liquid water oceans, and our planet was at approximately the same distance from the Sun that it is today. The planet was still spinning on its axis, but was doing so much more quickly: at about three-to-four times its current rotation rate. As the planet rotated on its axis and revolved around the Sun, ices froze and melted, precipitation occurred, and reservoirs of fresh water collected, in addition to the salty oceans.
From a chemistry-based perspective, however, that’s where the similarities end. As volcanic activity occurred, gases such as hydrogen sulfide, methane, ammonia, nitrogen, and carbon dioxide were released and/or formed. Energy gradients existed in many environments, driven by geothermal and solar energy inputs. And the amino acids that formed, including those that were part of Earth’s crust and mantle as well as those created by chemical reactions, all existed amidst a fantastically large ocean of possibilities, from hydrothermal vents to fields of heated, fresh water to tide pools and the shallows of continental shelves.
When we think about life, we normally think about all of the components possessed by all extant life today, including:
- a membrane for regulating what comes in and goes out,
- some sort of nucleic acid, like RNA or DNA, for encoding the vital information necessary to an organism’s functioning,
- some way of either producing or consuming its own food/energy,
- and the capacity to reproduce itself, generating offspring that are similar, if not identical, to the parent organism.
But all of these features, together, are likely far too complex and evolved to describe the very first organisms that arose on our planet. What likely happened first — although it’s still debated, and likely will be until we uncover a definitive answer — was that amino acids, in some sort of aqueous environment with an energy gradient, started bumping into one another. As things heat up, bonds break; as they cool down, bonds form. As amino acids bind together, they form peptide chains, and everything that occurs is driven, in some sense or other, by chance events.

In an aqueous environment, these amino acids also frequently encounter ions: positively charged ones like sodium, potassium, calcium and magnesium, and negatively charged ones like fluoride, hydroxide, and chloride, among others. Some peptides, if you add an ion to them, function like an enzyme. As different peptides interact with one another, uncountably large possibilities arise.
Bonds are broken by heat and energy, and are re-formed when then cool again, often in novel configurations. When you form bonds, you make new molecules, and when you break them apart in novel ways, you open up the possibility of forming molecules that have never been made before.
This occurred over and over, likely for tens or hundreds of millions of years, and some of the proteins that formed had the ability to perform specific tasks. Perhaps some were good at hoarding resources, like other peptides. Perhaps some were good at splitting other molecules apart and liberating energy from them when they did so. Perhaps some were good at making copies of themselves. Whatever the case, even though we have no idea what it looked like, something, at some point, “stuck,” meaning that it successfully took that first critical step from non-life toward becoming what we consider to be “alive.”
But what was it, specifically, that happened to differentiate Earth from all the “dead” worlds where no life ever emerged?
- Was there one specific protein or enzyme that was a critical intermediary toward the formation of life, and once formed, practically a guarantee of life?
- Were there multiple “false starts” that could have potentially given rise to life on our world, but were wiped out by a random event, like an ocean wave, a thermal current, or a lightning strike?
- Would the same protein that led to life’s eventual success here on Earth, whatever it was, have failed in an environment that was just a few degrees hotter or colder, with a slightly different pH, or that folded into a slightly different configuration?
In a situation such as this, we have to recognize that we are inextricably hindered by our bias toward life evolving when considering the question of how life could have evolved from non-life. We are stuck in an “n = 1” situation. If we discover a second, independent (i.e., not also descended from Earth-originating life, which could be the case, for example, if we discover past or extant primitive life on Mars) example of life originating from non-life, we can be confident this is not an ultra-rare process throughout the cosmos. But as long as we know of only us, it’s not a foregone conclusion to assume that life really is ubiquitous.

Sure, we can consider environments that are similar to and different from early Earth in a multitude of ways. Perhaps
- a colder, smaller planet, like Mars,
- a warmer, more energetic planet, like Venus,
- a world with liquid methane, rather than water, oceans, like Titan,
- a frozen world with a subsurface ocean, like Enceladus or Triton or Pluto,
- or a water-rich world that experiences significant tidal heating, like Europa,
could provide an environment where life can arise, survive, and thrive as well. Quite possibly, there may even be environments out there that are more conducive to the origin of life than even early Earth was.
Perhaps a planet or moon around a K-type or M-type star, both smaller, redder, and longer-lived than our Sun, would do the job? Perhaps a planet where life arises and then gets struck by a large object, like an asteroid, would seed other planets, moons, and even other star systems with that life? And perhaps, quite simply, if we rewound the clock to 4.5 billion years ago and made 1,000,000 identical copies of Earth, we could imagine how many of them would develop life. Would it be all 1,000,000 of them? A few thousand of them? Hundreds? Dozens? Just one? Or could life arising be such a rare occurrence that Earth is the outlier; that even in a million identical “early Earths,” not a single one would develop life?

This is the fundamental problem facing us. We are, in a very real sense of the word, the “winners” of a cosmic lottery. Despite all of the opportunities for failure at who-knows-how-many critical stages, life arose, survived, and thrived on our planet: an unbroken chain for some ~4 billion years. We know that we won the lottery, but we don’t know what occurred to lead to our victory. We don’t know what the odds of winning were, and we don’t know what the other prizes in the game were, or what the odds of winning them were.
- Is life common, and is practically every planet we’ve labeled as “potentially habitable” a place where life actually arose at some point?
- Are there places where life’s emergence is all but inevitable, but due to patterns that life can get “stuck” in, it doesn’t evolve beyond a certain, primitive stage?
- Is life too fragile to be common?
- Is life common, but is complex, differentiated life rare?
- Is it easy for life to arise, but difficult to survive through the billions of years of mass extinctions that occur?
All of these options, plus many more, remain viable possibilities.
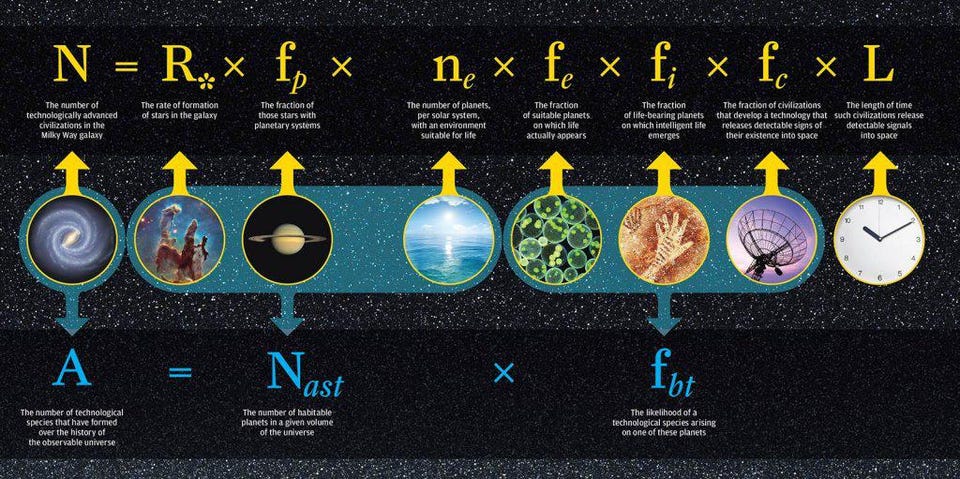
In all the cosmos, we can make excellent estimates at the number of lottery tickets our Universe provides us with for life to develop. What we aren’t quite so good at, given our current state of scientific knowledge, is knowing what the available prizes in the lottery are, or what the odds are of winning any one of them. One fascinating aspect to consider is this: life as we know it, here on Earth, might not even be the “grand prize” in the cosmic lottery.
Perhaps somewhere, under some conditions we might even consider too exotic for life, there are living creatures completely unlike us, better suited to surviving, thriving, and building an interstellar civilization in the cosmos. Perhaps they’re not made out of familiar ingredients like proteins, but something else entirely, like a crystalline or metallic set of structures. Despite the fact that we haven’t received any “positive” signals from SETI, that we haven’t found robust evidence for life on other worlds in our Solar System, or detected any inhabited planets around other stars just yet, we have every reason to keep searching. After all, absence of evidence is not evidence of absence, and we have no right to expect that life — of any type or complexity — is going to be easy to find.
But it is possible, and it occurred at least once. If we can find a second example, we’ll have every reason to expect that the Universe really is teeming with life. At that point, it simply becomes a numbers game: what are the odds, and what are the various prizes for winning?