Is The Matter In Our Universe Fundamentally Stable Or Unstable?

If we waited long enough, would even protons themselves decay?
There are certain things in the Universe that, if you leave them alone for long enough, they’ll eventually decay away. Other things, no matter how long we wait, have never been observed to decay. This doesn’t necessarily mean that they’re stable, only that if they’re unstable, they live longer than a certain measurable limit. While a large number of the particles — both fundamental and composite — are known to be unstable, there are a select few that appear to be stable, at least so far, to the precision we’ve been able to measure.
But are they truly, perfectly stable, destined never to decay even as the cosmic clock runs forward for all eternity? Or, if we could wait long enough, would we eventually see some or even all of those particles eventually decay away? And what does it mean for the Universe if a previously-thought-to-be-stable atomic nucleus, an individual proton, or even fundamental particles like the electron, a neutrino, or the photon turn out to decay? Here’s what it would mean if we lived in a Universe where our matter was fundamentally unstable.
It’s actually a relatively novel idea that any form of matter would be unstable: something that only arose as a necessary explanation for radioactivity, discovered in the late 1800s. Materials that contained certain elements — radium, radon, uranium, etc. — appeared to spontaneously generate their own energy, as though they were powered by some sort of internal engine inherent to their very nature.
Over time, the truth about these reactions was uncovered: the nuclei of these atoms were undergoing a series of radioactive decays. The three most common types were:
- α (alpha) decay: where an atomic nucleus spits out an α-particle (with 2 protons and 2 neutrons), moving down 2 elements on the periodic table,
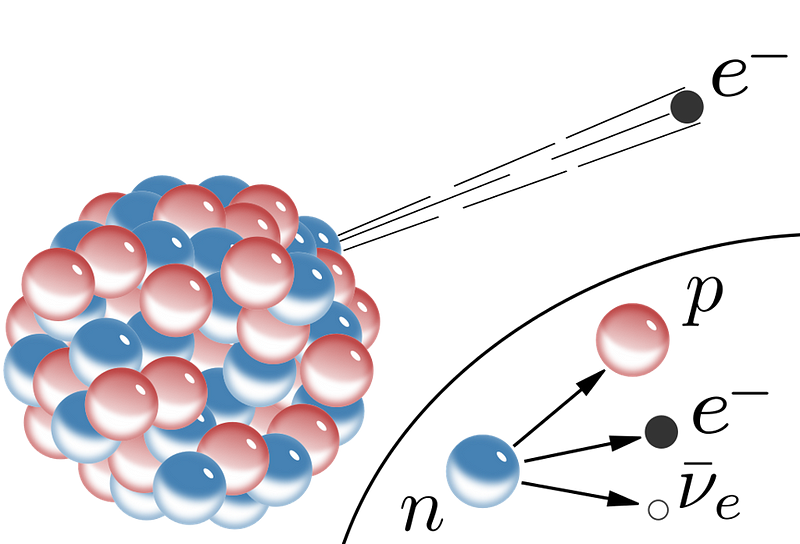
- β (beta) decay: where an atomic nucleus converts a neutron into a proton while spitting out an electron (a β-particle) and an anti-electron neutrino, moving up 1 element on the periodic table,
- γ (gamma) decay: where an atomic nucleus, in an excited state, spits out a photon (a γ-particle), transitioning to a lower-energy state.
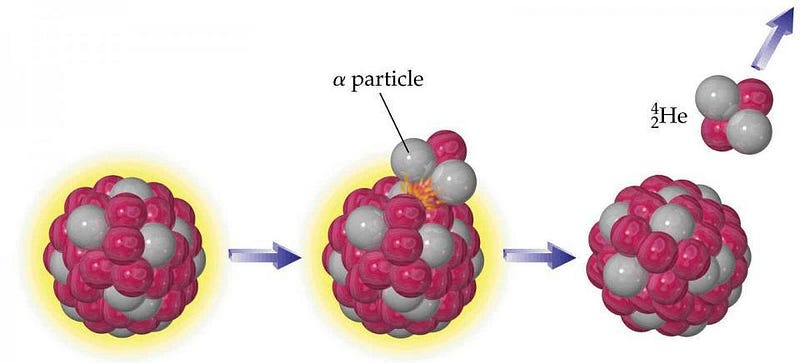
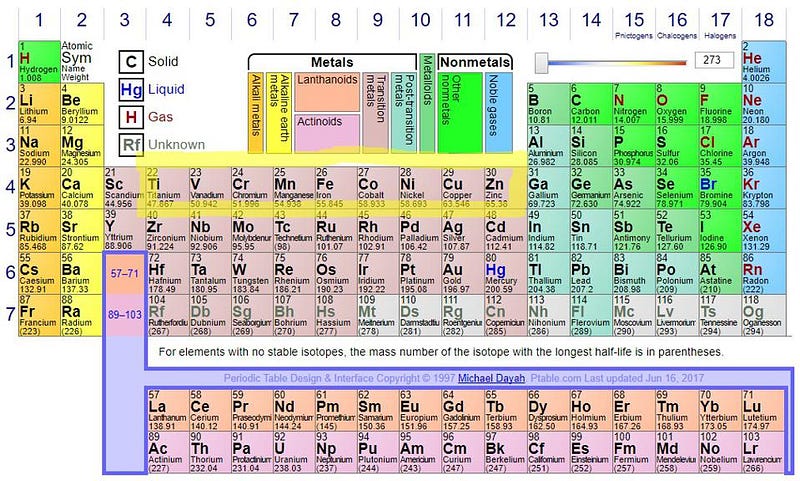
At the end of these reactions, the total mass of what’s left over (the products) is always less than the total mass of what we began with (the reactants), with the remaining mass converted into pure energy via Einstein’s famous equation, E = mc². If you learned about the periodic table prior to 2003, you probably learned that bismuth, the 83rd element, was the heaviest stable element, with every element heavier than that undergoing some form of radioactive decay (or decay chain) until a truly stable element is reached.
But in 2003, scientists discovered that every single isotope of bismuth is inherently unstable, including the abundant, naturally occurring bismuth-209. It’s extremely long-lived, with a half-life of around ~10¹⁹ years: approximately one billion times the age of the present Universe. Since that discovery, we now report that lead, the 82nd element, is the heaviest stable element. But given enough time, it’s possible that it will decay, too.
The reason that radioactive decays occur wasn’t well understood for many decades after the discovery of radioactivity: it’s an inherently quantum process. There are certain conservation rules that are an inextricable part of the laws of physics, as quantities like energy, electric charge, and linear and angular momentum are always conserved. That means, if we were to measure those properties for both the reactants and the products (or the physically possible products) of any candidate reaction, they must always be equal. These quantities cannot be spontaneously created or destroyed; that’s what it means to be “conserved” in physics.
But if there are multiple configurations that are allowed that obey all of those conservation rules, some of them will be more energetically favorable than others. “Energetically favorable” is like being a round ball on top of a hill and rolling down it. Where will it come to rest? At the bottom, right? Not necessarily. There can be many different low-points where the ball can wind up, and only one of them will be the lowest.
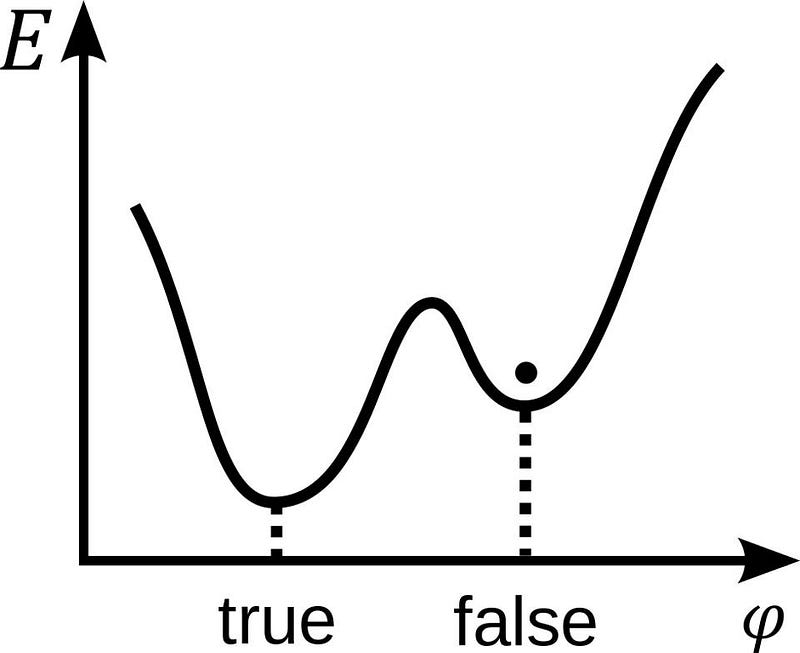
In classical physics, if you get trapped in one of these “false minima,” or a low-point that isn’t the lowest possible configuration, you’ll be stuck there unless something comes along to give that ball enough energy to rise above the boundaries of the pit it’s in. Only then will it have the opportunity to begin its descent down the hill anew, with the potential to eventually make it to a lower-energy configuration, possibly winding up in the lowest-energy (ground) state of all.
But in quantum physics, you don’t need to add energy for that transition to become possible. Instead, in the quantum Universe, it’s possible to spontaneously jump from one of those false minimum states to a lower-energy configuration — even directly into the ground state — without any external energy at all. This phenomenon, known as quantum tunneling, is a probabilistic process. If the laws of nature don’t explicitly forbid such a process from occurring, then it most definitely will. The only question is how long it will take.
In general, there are a few main factors that determine how long an unstable (or quasi-stable) state will last.
- What is the energy difference between the reactants and the products? (Bigger differences, and bigger percentage differences, translate to shorter lifetimes.)
- How suppressed is the transition from your current state to the final state? (I.e., what’s the magnitude of the energy barrier?)
- How many “steps” does it take to get from the initial state to the final state? (Fewer steps lead to a more likely transition.)
- And what is the nature of the quantum pathway that gets you there?
A particle like a free neutron is unstable, as it can undergo β decay, transitioning to a proton, an electron, and an anti-electron neutrino. (Technically, one of the down quarks inside β-decays into an up quark.) A different quantum particle, the muon, is also unstable and also undergoes β-decay, transitioning to an electron, an anti-electron neutrino, and a muon neutrino. They’re both weak decays, and both mediated by the same gauge boson. But because the products of neutron decay are 99.9% the mass of the reactants, while the products of muon decay are only ~0.05% of the reactants, the muon’s mean lifetime is measured in microseconds, while a free neutron lives for about ~15 minutes.
Measuring unstable particles individually is an excellent method for determining their properties so long as they’re short-lived compared to human timescales. You can observe them one-at-a-time and see how long they last until they eventually decay away. But for particles that live for extremely long times — longer even than the age of the Universe — that approach won’t work. If you took a particle like bismuth-209, and waited for the entire age of the Universe (~10¹⁰ years), there’s less than a 1-in-a-billion chance that it would decay. It’s a terrible approach.
But if you took an enormous number of bismuth-209 particles, like Avogadro’s number of them (6.02 × 10²³), then after a year a little more than 30,000 of them would decay. If your experiment was sensitive enough to measure that tiny change in the atomic composition of your sample, you’d be able to detect and quantify just how unstable bismuth-209 is. This idea was a critical test for an important idea in particle physics back in the 1980s: grand unified theories.

In our current, low-energy Universe, we have four fundamental forces: the gravitational force, the electromagnetic force, and the strong and weak nuclear forces. At high energies, two of those forces — the electromagnetic force and the weak nuclear force — unify and become a single force: the electroweak force. At still higher energies, based on important ideas from group theory in particle physics, it’s theorized that the strong nuclear force unifies with the electroweak force. This idea, called grand unification, would have important consequences for a vital building block of matter: the proton.
Under the Standard Model alone, there’s no good pathway for the proton to decay; its lifetime should be so long that if we monitored every proton in the Universe for the lifetime of the Universe since the Big Bang, exactly zero of them should decay. But if grand unification is correct, then the proton should easily be able to decay into pions and (anti-)leptons, and should have a lifetime of “merely” ~10³⁰ years in the simplest model. That might seem unfathomably long, but physicists have a way to test this.
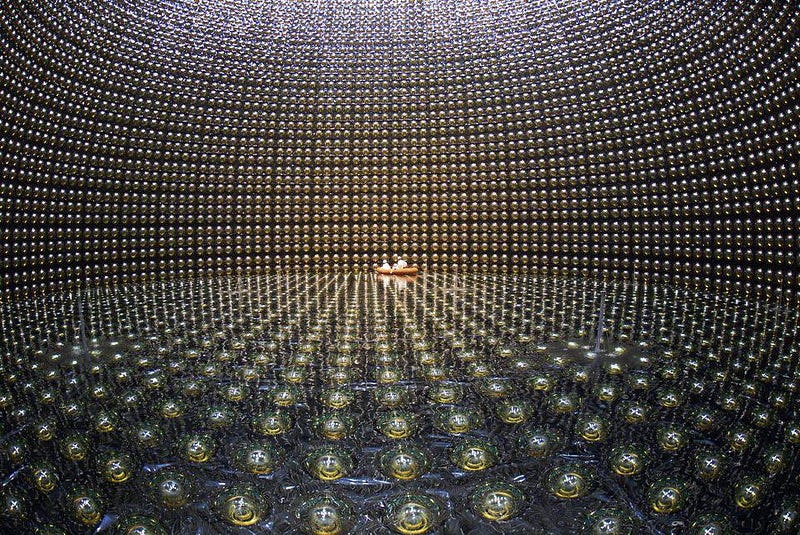
All you have to do is gather enough protons — such as from the hydrogen atoms in a water molecule — together in one place, and build a sensitive enough suite of detectors to identify the telltale signal that would emerge if protons decayed. If you get 10³⁰ of them together and wait for a year, you should be able to measure their half-life if it’s shorter than 10³⁰ years, and place a lower limit on their lifetime otherwise. After decades of these experiments, combined with the information we learn about proton lifetimes from neutrino detector experiments, we now know the proton’s lifetime can be no shorter than around ~10³⁵ years.
That tells us that the simplest grand unified theories cannot reflect our reality, but it doesn’t tell us whether the proton is truly stable or not. Similarly, “stable” atomic nuclei may someday decay; electrons, neutrinos, and photons may someday decay; even gravitational waves or space itself may not be eternal. Some of our strongest constraints on beyond-the-Standard-Model physics come from the non-observation of these and other decays. To the limits of what we’ve measured, most of the Universe’s components appear stable.
But is the matter in our Universe truly stable in some form, or will it all eventually — if we wait for arbitrarily long times — decay in some way? It’s important to remember that what we’re measuring with our experiments is limited to how we’re performing our experiments.
For example, a free neutron has a mean lifetime of ~15 minutes, but a neutron in a neutron star has enough binding energy that it’s entirely stable: it can never decay. Similarly, it’s possible that protons or certain atomic nuclei really are intrinsically unstable, but because we’re measuring them as they are bound in atoms and molecules, we see them as stable. Our conclusions are only as good as the experiments used to reach them.

Nevertheless, the fact that we’ve measured the stability of so many fundamental and composite particles gives us the strongest constraints of all, in many ways, on possible modifications to the Standard Model. Simple models of grand unification are ruled out. Many supersymmetric theories are completely dead. Other ideas that introduce new particles, including technicolor theories and theories involving extra dimensions, are restricted by the observed stability of the matter in our Universe.
Even though the ultimate fate of the matter in our Universe has yet to be determined, the wiggle room is already narrower than many of the greatest ideas that 20th and 21st century physicists have been able to concoct. We may not know everything about what the Universe is, but it’s impressive how much we know about what the Universe isn’t.
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.





