Ask Ethan: How cold does it get in space?

- No matter where you go in the Universe, there are some sources of energy you simply can’t get away from, like the cosmic microwave background radiation left over from the hot Big Bang.
- Even in the deepest depths of intergalactic space, hundreds of millions of light-years away from any stars or galaxies, this radiation still remains, heating all things up to 2.725 K.
- But there are places in the Universe, somehow, that get even colder than that. Here’s how to make the coldest places in all the cosmos.
When we talk about the depths of space, we get this picture in our heads of emptiness. Space is barren, sparse, and largely devoid of anything, save for the “islands” of structure that permeate the Universe. The distances between planets is vast, measured in millions of kilometers, and those distances are relatively small compared to the average distance between stars: measured in light-years. Stars are clustered together in galaxies, where they’re joined by gas, dust, and plasma, although the individual galaxies themselves are separated by even greater lengths.
Despite the cosmic distances, however, it’s impossible to ever be totally shielded from other sources of energy in the Universe. What does that mean for the temperatures of deep space? These questions were inspired by the inquiry of Patreon supporter William Blair, who asks:
“I discovered this little gem in [Jerry Pournelle’s writings]: “The effective temperature of outer space is about -200 degrees C (73K).” I don’t think that’s so, but I figured you would know for sure. I figured it would be 3 or 4 K… Could you enlighten me?”
If you search online for the temperature of space, you’ll come across a variety of answers, ranging from just a few degrees above absolute zero to more than a million K, depending on where and how you look. When it comes to the question of temperature in the depths of space, the three cardinal rules of real estate most definitely apply: location, location, location.
The first thing we have to reckon with is the difference between temperature and heat. If you take a certain amount of heat energy and add it into a system of particles at absolute zero, those particles will speed up: they will gain kinetic energy. However, the same amount of heat will change the temperature by very different amounts depending on how many particles there are in your system. For an extreme example of this, we need to look no further than Earth’s atmosphere.
As anyone who’s ever climbed a mountain can attest, the higher you go in elevation, the colder the air around you gets. This isn’t because of a difference in your distance from the light-emitting Sun or even from the heat-radiating ground of the Earth, but rather because of a difference in pressure: with lower pressure, there’s less heat and fewer molecular collisions, and so the temperature drops.
But as you go to extreme altitudes — into Earth’s thermosphere — the highest-energy radiation from the Sun can split molecules apart into individual atoms, and then kick the electrons off of those atoms, ionizing them. Even though the density of particles is tiny, the energy-per-particle is very high, and these ionized particles have tremendous difficulty radiating their heat away. As a result, even though they carry only a minuscule amount of heat, their temperature is tremendous.
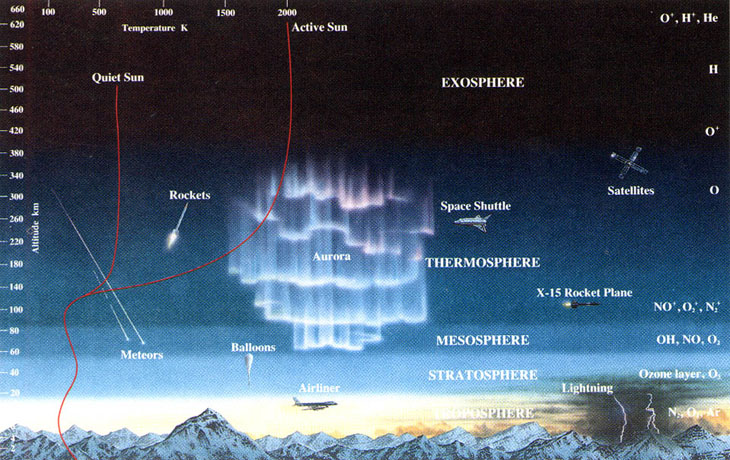
Rather than rely on the temperature of the particles in any particular environment themselves — since that temperature reading will depend on the density and type of particles that are present — it’s a more useful question to ask, “If I (or any object made of normal matter) were hanging out in this environment, what temperature would I eventually reach when equilibrium was obtained?” In the thermosphere, for example, even though the temperature varies between 800-1700 °F (425-925 °C), the truth of the matter is that you would actually freeze to death extremely quickly in that environment.
When we head to space, therefore, it’s not the ambient temperature of the environment surrounding us that’s important, but rather the sources of energy that are present, and how good of a job they do at heating up the objects they come into contact with. If we went straight up until we were in outer space, for example, it would be neither the heat radiated from the Earth’s surface nor the particles from Earth’s atmosphere that dominated our temperature, but rather the radiation coming from the Sun. Even though there are other sources of energy, including the solar wind, it’s the full spectrum of light from the Sun, i.e., electromagnetic radiation, that determines our equilibrium temperature.

If you were located in space — like every planet, moon, asteroid, and so on — your temperature would be determined by whatever value you possessed where the total amount of incoming radiation equaled the amount of radiation you emitted. A planet with:
- a thick, heat-trapping atmosphere,
- that’s closer to a source of radiation,
- that’s darker in color,
- or that generates its own internal heat,
is generally going to have a higher equilibrium temperature than a planet with the opposite set of conditions. The more radiation you absorb, and the longer you retain that energy for before re-radiating it away, the hotter you’ll be.
However, if you were to take the the same object and place it at different locations in space, the only thing that would determine its temperature is its distance from all the different sources of heat within its vicinity. No matter where you are, it’s your distance from what’s around you — stars, planets, clouds of gas, etc. — that determines your temperature. The greater the amount of radiation that’s incident on you, the hotter you get.
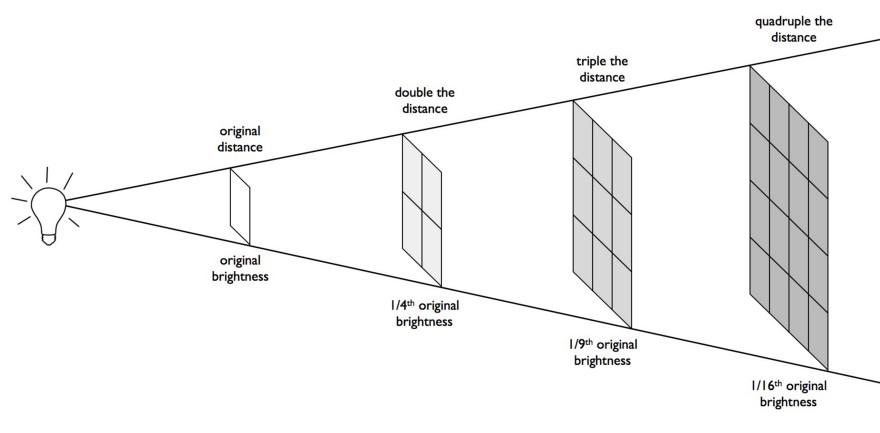
For any source that emits radiation, there’s a simple relationship that helps determine how bright that source of radiation appears to you: the brightness falls off as one over the distance squared. That means:
- the number of photons impacting you,
- the flux incident on you,
- and the total amount of energy absorbed by you,
all decrease the farther away you are from a radiation-emitting object. Double your distance, and you’ll receive just one-quarter of the radiation. Triple it, and you’ll receive just one-ninth. Increase it by a factor of ten, and you’ll get merely one-hundredth of the original radiation. Or you can travel a thousand times farther away, and a meagre one-millionth of the radiation will strike you.
Here at Earth’s distance from the Sun — 93 million miles or 150 million kilometers — we can calculate what the temperature would be for an object with the same reflectivity/absorption spectrum as Earth, but with no atmosphere to retain heat. The temperature of such an object would be -6 °F (−21 °C), but since we don’t like dealing with negative temperatures, we more frequently speak in terms of kelvin, where this temperature would be ~252 K.
At most locations in the Solar System, the Sun is the primary source of heat and radiation, meaning that it’s the primary arbiter of temperature within our Solar System. If we were to place that same object that’s ~252 K at Earth’s distance from the Sun at the location of the other planets, we’d find that it’s the following temperature at:
- Mercury, 404 K,
- Venus, 297 K,
- Mars, 204 K,
- Jupiter, 111 K,
- Saturn, 82 K,
- Uranus, 58 K,
- and Neptune, 46 K.
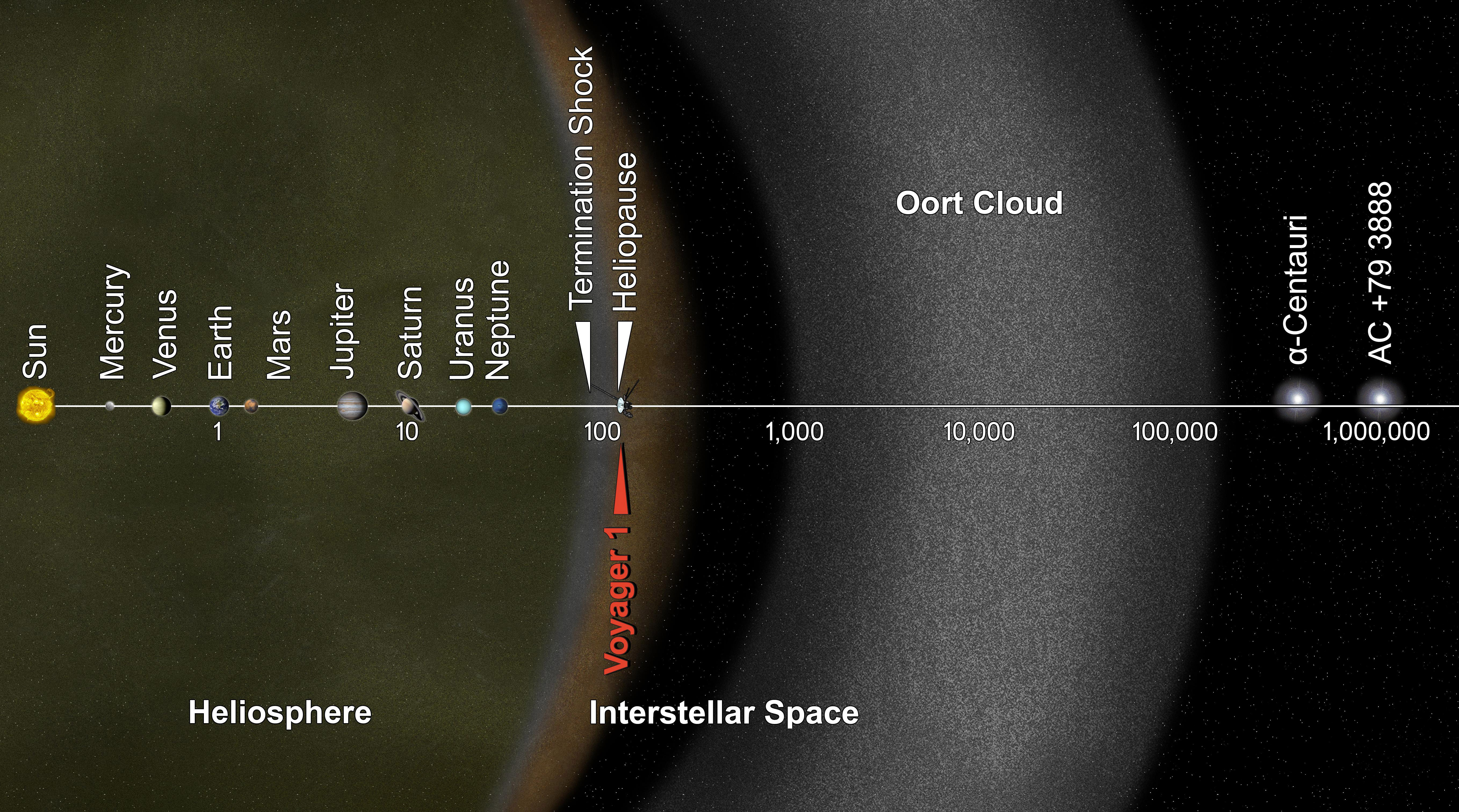
There’s a limit, however, to how cold you’re going to get by continuing to travel away from the Sun. By the time you’re more than a few hundred times the Earth-Sun distance away, or around ~1% of a light-year distant from the Sun, the radiation impacting you is no longer primarily coming from just one point source.
Instead, the radiation from the other stars in the galaxy, as well as the (lower-energy) radiation from the gases and plasmas in space, will start to heat you as well. As you get farther and farther away from the Sun, you’ll start to notice that your temperature simply refuses to drop below about ~10-20 K.
In between the stars in our galaxy, matter can be found in all sorts of phases, including solids, gases, and plasmas. Three important examples of this interstellar matter are:
- molecular clouds of gas, which will only collapse once the temperature within these clouds drops below a critical value,
- warm gas, mostly hydrogen, which zips around due to its heating from starlight,
- and ionized plasmas, which primarily occur near stars and star-forming regions, predominantly found near the youngest, hottest, bluest stars.
While plasmas can typically and easily reach temperatures of ~1 million K, and warm gas typically achieves temperatures of a few thousand K, the far-denser molecular clouds are usually cool, at ~30 K or less.
Don’t be fooled by these large temperature values, however. Most of this matter is incredibly sparse and carries very little heat; if you were to place a solid object made of normal matter into the spaces where this matter exists, the object would cool tremendously, radiating far more heat than it absorbs. On average, the temperature of interstellar space — where you’re still within a galaxy — sits at between 10 K and “a few tens” of K, depending on quantities like the density of the gas and the number of stars in your vicinity.

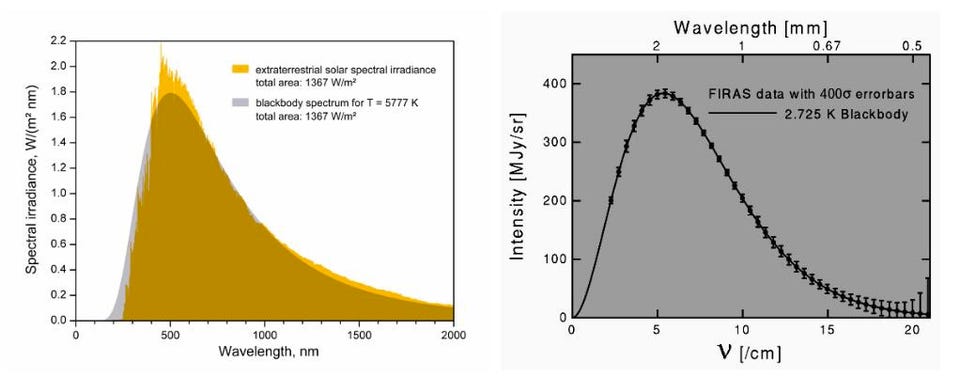
You’ve likely heard, quite correctly, that the temperature of the Universe is right around 2.7 K, however, a much colder value than you’ll find in most places throughout the galaxy. This is because you can leave most of these heat sources behind by going to the right location in the Universe. Far away from all the stars, away from the dense or even sparse clouds of gas that exist, between the tenuous intergalactic plasmas, in the most underdense regions of all, none of these sources of heat or radiation are significant.
The only thing left to contend with is the one unavoidable source of radiation in the Universe: the cosmic microwave background radiation, itself a remnant from the Big Bang. With ~411 photons per cubic centimeter, a blackbody spectrum, and a mean temperature of 2.7255 K, an object that was left in the depths of intergalactic space would still heat up to this temperature. At the lowest-density limits obtainable in the Universe today, 13.8 billion years after the Big Bang, this is as cold as it gets.
Only, there’s a mechanism by which the Universe, naturally, can finesse its way to even lower temperatures. Whenever you have a cloud of gas or a plasma, you have the option, regardless of its temperature, of rapidly changing the volume that it occupies. If you contract the volume rapidly, your matter heats up; if you expand the volume rapidly, your matter cools down. Of all the gas-and-plasma-rich objects that expand in the Universe, the ones that do so most quickly are red giant stars ejecting their outer layers: the ones that form preplanetary nebulae.
Of all of those, the coldest every observed is the Boomerang Nebula. Although there’s an energetic red giant star at its center, and there’s both visible and infrared light getting emitted from it in two giant lobes, the expanding material ejected from the star has cooled so rapidly that it’s actually below the temperature of the cosmic microwave background. Simultaneously, because of the density and opacity of the environment, that radiation cannot get in, enabling this nebula to remain at just ~1 K, making it the coldest naturally-occurring location in the known Universe. Quite likely, many preplanetary nebulae are also colder than the cosmic microwave background, meaning that within galaxies, there are occasionally places that are colder than the deepest depths of intergalactic space.

If we had easy access to the deepest depths of intergalactic space, building an observatory like JWST would have been a much easier task. The five-layer sunshield, which passively cools the telescope down to approximately ~40 K, would have been wholly unnecessary. The active coolant, which gets pumped and flows through the telescope’s interior, cooling the optics and the mid-infrared instrument all the way down to below ~7 K, would be redundant. All we’d have to do was place it in intergalactic space, and it would passively cool, all on its own, down to ~2.7 K.
Whenever you ask what the temperature of space is, you cannot know the answer without knowing where you are and what sources of energy are affecting you. Don’t be fooled by extremely hot but sparse environments; the particles there may be at a high temperature, but they won’t heat you nearly as much as you’ll cool yourself. Near a star, the star’s radiation dominates. Within a galaxy, the sum of starlight plus the radiated heat from gas determines your temperature. Far away from all other sources, the cosmic microwave background radiation dominates. And within a rapidly expanding nebula, you can achieve the coolest temperatures of all: the closest the Universe ever gets to absolute zero.
There’s no Universal solution that applies to everyone, but the next time you find yourself wondering about just how cold you’d get in the deepest depths of space, at least you’ll know where to look for the answer!
Send in your Ask Ethan questions to startswithabang at gmail dot com!