Ask Ethan: When Did The Universe Become Transparent To Light?

It happened more than once, and needed to. Here’s why.
If there’s one thing you can be certain about when it comes to outer space, it’s that it’s transparent, not opaque, to light. When you gaze up at a dark night sky, you aren’t restricted to seeing what’s merely in our atmosphere, in low-Earth orbit, in our Solar System, or even what’s in our galaxy. Instead — particularly if you have a tool to gather more light than your eye can take in in real-time — we can literally gaze across the Universe, seeing objects that are thousands, millions, or even billions of light-years away. All of this would be impossible if the Universe weren’t transparent to light.
But, at the same time, two other things are also true. First, we cannot see infinitely far away; there’s a limit to how far back we’re able to look. And second off, light comes in many different wavelength bands, and not every set of wavelengths is equally transparent to every other set. What, exactly, can we say about when the Universe became transparent to light? That’s what Barry McMahon wants to know, asking:
“[I] was confused by a statement [you made] about re-ionization which says that ‘over hundreds of millions of years the universe became transparent as its gas particles became charged or ionized.’ As I understand it, the universe was already transparent at this stage (transparency is associated with the recombination which occurred at a much earlier epoch when the universe had cooled sufficiently). Re-ionization did occur of course as stars and galaxies formed after a couple hundred million years, but the universe was so large by then and the free electrons so widely separated that they only rarely scattered photons. Thus the universe remained transparent, it did not become transparent… Do you agree?”
There are two important phases that did, in fact, occur, and both affected the ability of light to pass through the Universe: recombination and reionization. Here’s what you need to know to understand why the Universe is transparent today.
In the early stages of the hot Big Bang, the Universe is the least transparent it will ever be. Since it was hotter and denser long ago, all of the normal matter in the Universe was ionized, meaning there were plenty of free protons and electrons flying around, unable to form neutral atoms due to the high temperatures and energies. There are also photons — quanta of light — present as well, in great numbers and great densities.
When something is transparent to light, that means light passes right through it, with its path and properties largely unchanged by the objects it encounters. The early Universe, then, filled with fast-moving charged particles, is perhaps the ultimate example of a set of conditions that is not transparent to light. Photons have a large chance for interacting with particles, what we call a cross-section, if those particles are:
- electrically charged,
- energetic,
- and low in mass,
which is a set of parameters that fits one type of particle in particular extremely well: the electron.

In the early Universe, the electron is the primary reason the Universe isn’t transparent. Every photon that travels through space, no matter what direction it travels in, can only make it an extremely short distance before encountering an electron. You can think of an electron and a photon each as particles, and those particles have an energy-dependent cross-section, so the higher the energies of your particles, the greater the chance they’ll collide and scatter: going off in different directions from how they were moving initially.
However, you can also treat photons like waves, which is more intuitive to some people. Photons are electromagnetic waves, with oscillating in-phase electric and magnetic fields, and those fields will act on and accelerate any electron they encounter. If the electron changes momentum, then there needs to be an equal-and-opposite change in momentum somewhere else so that, overall, momentum is conserved. So however much you change the electron’s momentum by, you must change the photon’s momentum by an equal and opposite amount, and hence, the photon has to change direction.
This is why, when we plot out how a photon changes direction when it encounters electrons as a function of energy, we see that the energy is tremendously important for how much the photon gets deflected by in its encounter with the electron.
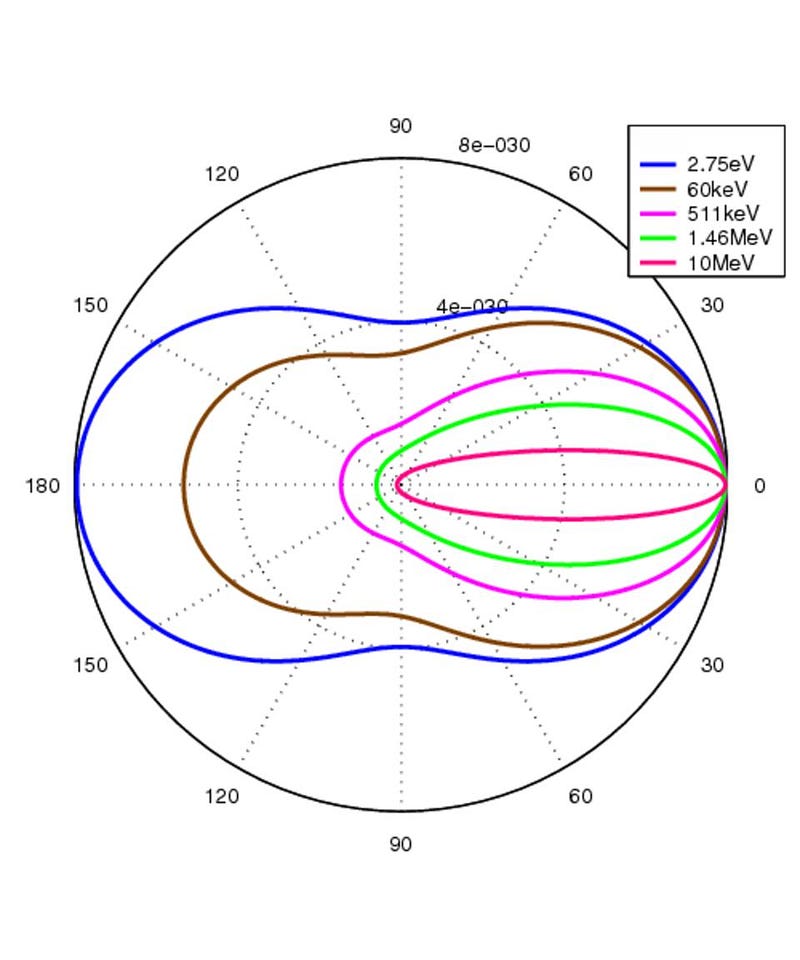
As long as there are ionized particles permeating all of space — which is definitely the case prior to the formation of stable, neutral atoms — photons cannot travel for even a second without encountering an electron and changing direction. These scattering events render the Universe opaque, in the sense that the light that comes in gets scattered and redirected, and these scattering interactions can also change the light’s energy/wavelength. For the first few hundred thousand years after the Big Bang, this occurs continuously for all photons, and the Universe remains opaque.
Opaque, in this context, doesn’t mean we couldn’t see anything if we were present back then, but rather that you can’t see anything from far away. There’s lots of reflected and re-emitted light coming at you from all directions at these early times, but if you examined where each photon was coming from since the previous interaction with an electron happened — where the point of “last scattering” occurred — you’d find that it was extremely close to you. In other words, you couldn’t see light from any object that was, well, an astronomical distance away from you.
But as the Universe cools below a critical temperature, about ~3000 K, the photons are now redshifted by the expanding Universe so thoroughly that there aren’t enough high-energy ones left to ionize the atoms that begin forming. For the first time, we can make stable, neutral atoms.
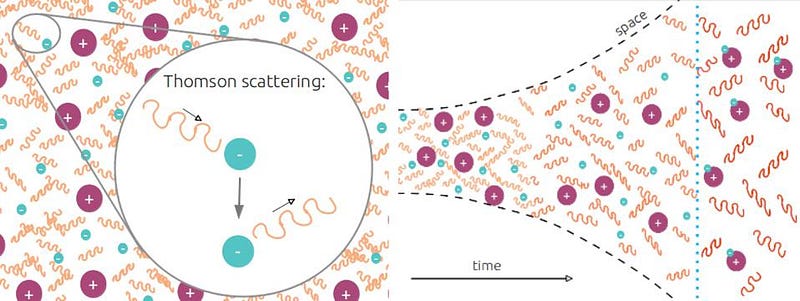
This is an important milestone, often called “recombination” by astrophysicists. The free electrons in the Universe have been trying to bind to the protons and other atomic nuclei that are floating around out there, but every time they do so, they’ve been kicked off by a high-enough-energy photon. They combine, they get ionized, and they try again: recombining. (Much later on in the Universe, when stars form, new stars ionize the atoms inside, and then those free electrons recombine with those ions to form atoms again, which gives “recombination” its name.) Although it’s a slow and gradual process taking more than 100,000 years, eventually it completes, and for the first time, the Universe is filled with neutral atoms and practically no more free electrons and ions.
That event changes the story tremendously for photons. When a photon encounters a free electron, it scatters with it: Compton scattering at high energies, Thomson scattering at low energies. Any electron it runs into will change its direction. But when that same photon encounters a neutral atom, it will only interact with it if the photon has just the right wavelength to cause a transition in the electron’s energy levels. Once these neutral atoms form, however, practically every photon is too low in energy — with too long a wavelength — to interact with those atoms. As a result, the photons no longer scatter, but simply pass through the now-neutral atoms as though they weren’t there at all. We call this free-streaming, as the photons are now unchanged except for the cosmological redshift that stretches their wavelength as they travel, and these photons continue to do exactly that even to the present day.
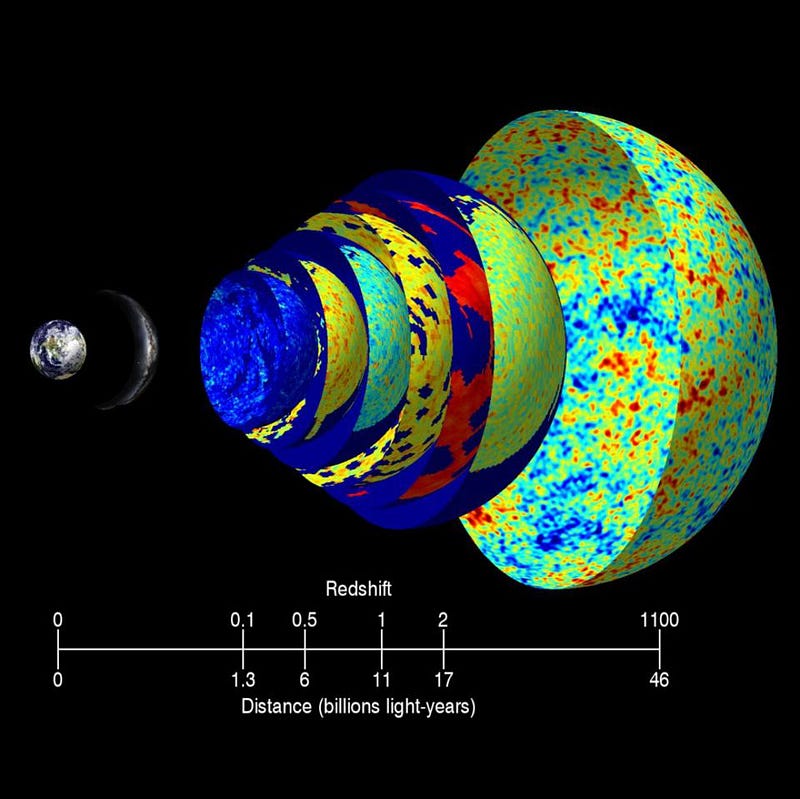
In this sense, the Universe becomes transparent when neutral atoms stably form and recombination occurs. Which is to say, the Universe becomes transparent to the photons that are left over from the Big Bang: what we observe today as the cosmic microwave background. At the time the Universe becomes neutral, most of these photons are in the “red” part of the visible light spectrum, while the neutral atoms have their electrons in the lowest-energy state, where they (mostly) absorb ultraviolet light.
As time goes on, the photons only get redshifted further and brought to lower energies: from visible light to infrared to microwave wavelengths, where they continue to free-stream through the Universe, even to the present day. The “surface of last scattering” for these photons occurred when the Universe was only 380,000 years old, on average: the last time that they scattered with a free electron.
But that’s when the Universe becomes transparent to the light that’s left over from the Big Bang. When we look out at the Universe with microwave eyes, that’s what we see: the Big Bang’s leftover glow, the cosmic microwave background. But when we look out with our own eyes, we see visible light: the light generated by stars. And that requires a whole different type of transparency, for reasons that are clear to see.

Out in the Universe today, you need look no further than the Milky Way itself to understand why these neutral atoms are absolutely terrible at being transparent to starlight. The Milky Way, if you’ve ever seen it, looks like a swath of faint, milky clouds with dark bands running through it, particularly towards the densest, most central region. Those dark bands are actually neutral matter — clouds of gas and dust — bound together through their own gravity. These clouds are partially clumped together into grains of a particular set of sizes, and in general, these dust grains will absorb light if its wavelength is the size of the grain or smaller, and won’t if the wavelength is longer.
These neutral atoms need to clump and gravitate before we can even form the very first stars in the Universe, meaning that anywhere we form stars, that star-forming region will be rife with and surrounded by this gas-and-dust. When the first stars turn on, that’s the first thing the starlight is going to encounter: neutral atoms, clumped together, that’s opaque to the light being emitted by the stars. The earliest stars in the Universe, in addition to being very different from the stars we have today, composed solely of hydrogen and helium, are also created in dense environments from which the very starlight they’re creating cannot escape.

But time changes all things, including the status of these neutral atoms. As matter starts to clump together and form gravitationally bound structures, we get regions that are much denser than average. Correspondingly, that matter has to come from somewhere, so the surrounding regions of average and below-average densities preferentially give up their matter to these denser regions. Where the densities climb high enough, stars form, and starlight — for the first time — not only gets created, but starts slamming into the neutral matter around them.
Now, this is where the second type of opacity comes into play: the Universe is transparent to the leftover photons from the Big Bang, but not to the photons created by stars. In particular, most of the light generated is ultraviolet and visible light: short-wavelength, high energy light, easily absorbed by the realistic dust grains present. But the ultraviolet light has a special property that allows it to begin changing the situation: it has enough energy to ionize the atoms that it comes into contact with, kicking many of the electrons off of their atoms. When enough stars get created, the radiation can actually break through this sheath of neutral matter, ionizing it and sending starlight — for the first time — out into the Universe beyond.
Early on, there are only a few pockets of star-formation that occur. Additionally, at relatively early times in the Universe, it’s still relatively small in size, having not had sufficient time to expand to larger scales and dilute (in terms of density) to fewer particles-per-unit-volume. This means that many of the atoms that get ionized at very early times from the formation of the first stars can become neutral again. Star formation occurs in bursts and waves, so dense regions can become mostly ionized, then mostly neutral, and then mostly ionized again.
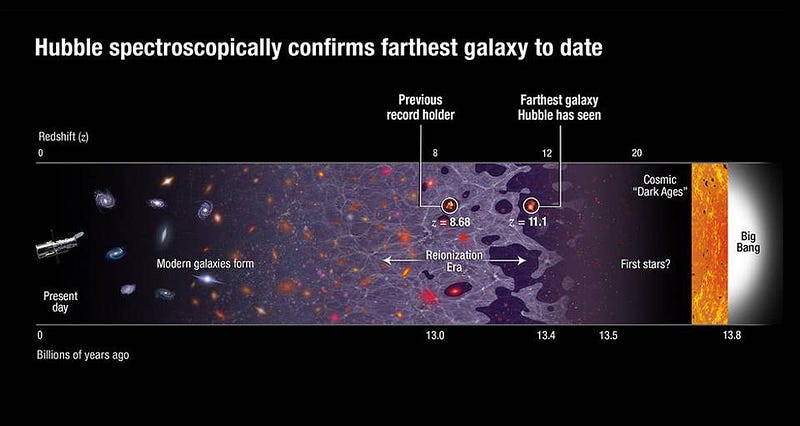
It takes a long amount of time, and the sustained production of new, massive, ultraviolet-emitting stars to ionize not only the matter in the densest regions, but also the atoms that still lurk in the space between stars and galaxies: the intergalactic medium. Although the very first stars might turn on between 50–100 million years after the Big Bang and the first big waves of star formation might happen just 200–250 million years after the Big Bang, small amounts of neutral matter can go a long way. It isn’t until ~550 million years after the Big Bang that the final ~1% of neutral matter that’s left — the final atoms in the intergalactic medium — get fully ionized, allowing starlight to pass through without being impeded by the gas and dust.
“Wait a second,” I can hear you objecting. “I thought that ionized atoms made free electrons, and that free electrons were the enemy of photons because they caused scattering!”
And to that objection, I respond that you are correct, but that it’s not just about the state of matter you’re in and the photon energy, but also about the density of particles present. In the space between galaxies — the intergalactic medium — there’s only about one electron per cubic meter of space, and these photons aren’t substantially affected by electrons at these low densities. There are simply too many of them (photons) for the number of electrons present.
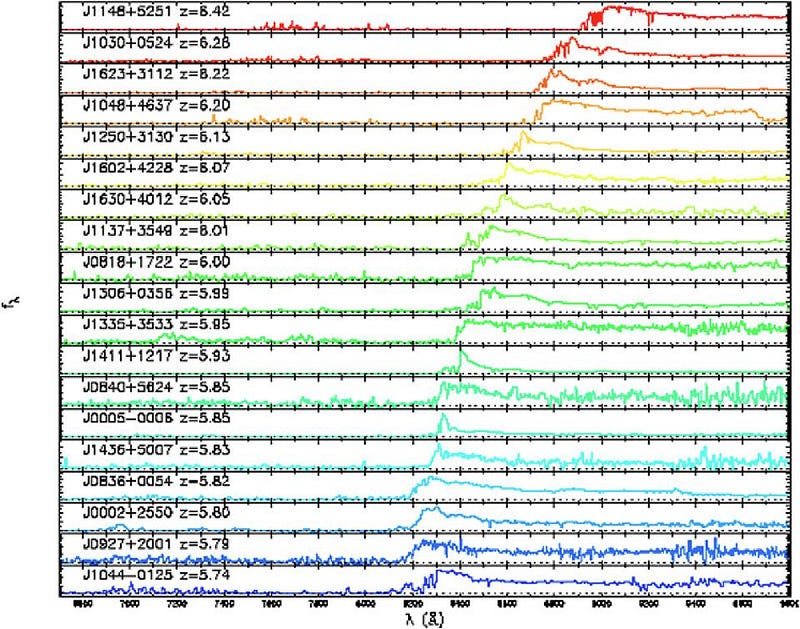
However, there is a limit to how far back we can look, as in all directions, there’s a “wall” in time where there are suddenly large densities of neutral atoms. In rare cases, it’s because there are nebulae — dense clumps of matter — intervening. But in most cases, we can look back about 30 billion light-years, give or take, before we find that there wasn’t quite enough starlight created just yet to fully reionize the Universe, and hence a lot of the emitted light gets absorbed before it can ever reach us. The transition is most abrupt in the quasar data, which shows the appearance (or lack of appearance) of these neutral, absorptive atoms in their spectra: the Gunn-Peterson trough.
When you put all that we’ve learned together, it not only paints a fascinating picture, but opens up the Universe — if we look at it in just the right way — with the incredible potential for pushing the frontiers as never before. The Universe starts out hot, dense, and ionized, meaning that photons from the Big Bang constantly scatter off of electrons, which they do until the Universe forms neutral atoms 380,000 years after the Big Bang. Only then can those much-cooler photons now free-stream.
However, the neutral atoms gravitate and clump together, where visible and ultraviolet light cannot pass through them in these dense environments. Only ~550 million years later, when enough stars produce enough high-energy radiation to ionize the whole intergalactic medium, is the Universe transparent to starlight.
But this means that if we look in longer wavelengths of light, the Universe won’t appear quite as opaque, even at those early times between recombination and the end of reionization. Infrared and even radio light can always pass right through, giving the James Webb Space Telescope and other, even longer-wavelength observatories, a chance to find the stars and galaxies whose visible starlight gets swallowed by the intervening matter. Transparency, as always, depends not only when you look, but also how: in which wavelengths of light.
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.





