60 Years of Starstuff

How humanity discovered where our elements come from.
This article was written by physicist Paul Halpern of the University of the Sciences in Pennsylvania. Paul is author of the new book The Quantum Labyrinth: How Richard Feynman and John Wheeler Revolutionized Time and Reality.
“You couldn’t be here if stars hadn’t exploded, because the elements — the carbon, nitrogen, oxygen, iron, all the things that matter for evolution and for life — weren’t created at the beginning of time. They were created in the nuclear furnaces of stars, and the only way for them to get into your body is if those stars were kind enough to explode…” –Lawrence Krauss
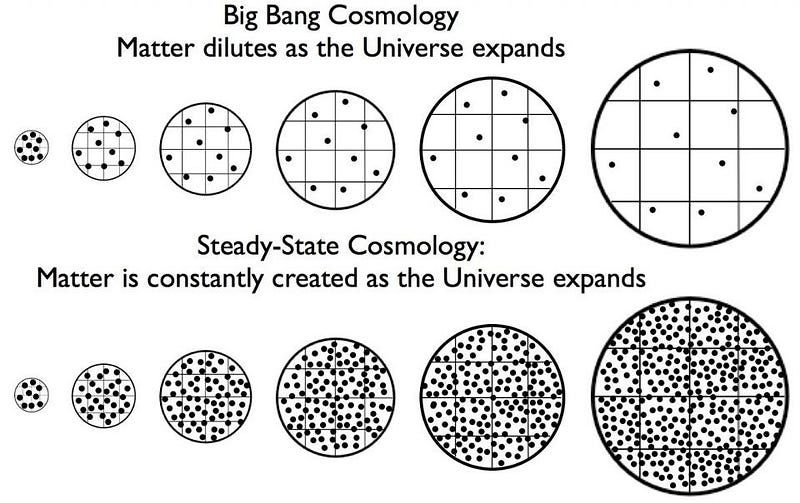
In science, you don’t have to get everything right in order to get the most incredible things correct. Sometimes good ideas emerge from a failed paradigm. An excellent example of both is the groundbreaking stellar nucleosynthesis paper (creation of complex nuclei from simpler ones) paper, published in 1957, known simply as B2FH, after the initials of the four authors. For the first time, it offered a successful model of element formation. It was designed to avoid the need for a Big Bang, and to support an alternative explanation called Steady State theory. Today, while Steady State theory is a relic of the past, stellar nucleosynthesis complements the Big Bang theory in a successful, comprehensive explanation of how all of the elements in the universe were built from elementary particles.
It is a curious fact of history that the first time an astronomer used the term “Big Bang” to describe the early stages of the Universe, he meant it derisively. Cambridge researcher Fred Hoyle (the “H” in the pivotal paper), who coined the expression in a 1948 BBC radio interview, thought the idea of all the matter in the universe emerging at once, like the sudden burst of a colossal cosmic piñata, to be patently ridiculous.

While he believed in an expanding cosmos, he thought it would last forever in a Steady State of near sameness, with a slow trickle of fresh matter filling the gaps — similar to a tailor adding new buttons to a suit altered for a growing child.
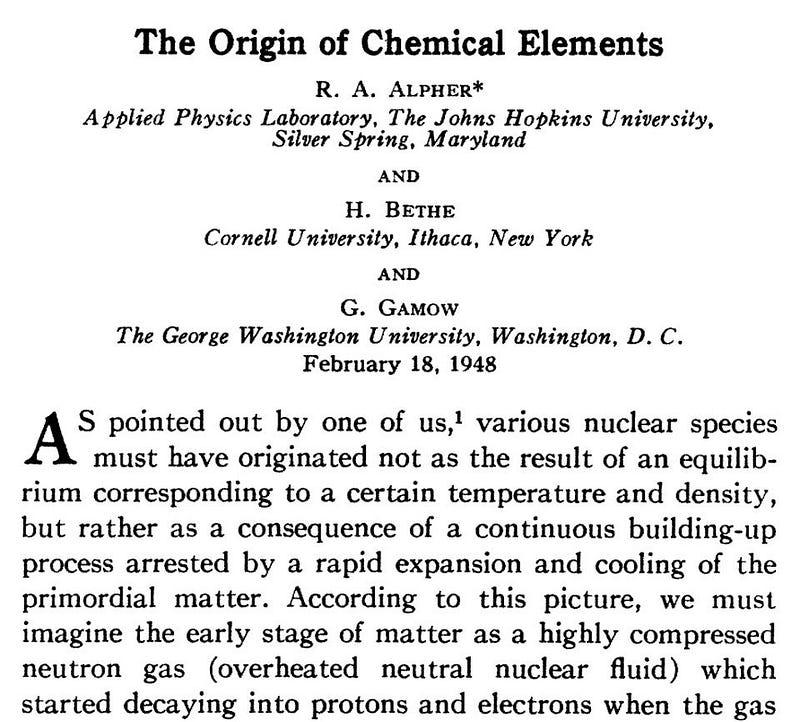
One of the main problems with Hoyle’s Steady State scheme, co-developed with Thomas Gold and Herman Bondi, was explaining how the cold, elementary particles gradually seeping into space could transmute into higher elements. In that domain, the Big Bang theory, at first, claimed to have all the answers. George Gamow, along with his student Ralph Alpher, purported to explain the entirety of element creation through Big Bang nucleosynthesis. That is, they argued that the fiery cauldron of the Big Bang forged all of the natural chemical elements, from hydrogen up through uranium, out of the simpler proton and neutron building blocks. They published their work in a key paper “Origin of the Chemical Elements,” that appeared in April 1948.

Gamow had a wonderful sense of humor and loved to play practical jokes. Noting that Alpher’s name and his resembled the first and third letters of the Greek alphabet, alpha and gamma, when he submitted the paper, he decided to add physicist Hans Bethe’s name, which sounded like beta, as the second author. Bethe had almost nothing to do with the paper. However, he was an expert in nucleosynthesis, so the idea wasn’t as crazy as it sounded. Hence the seminal article is universally known as the alpha-beta-gamma paper. (When another graduate student Robert Herman joined the team, Gamow jokingly suggested that he change his name to “Delter,” just to fit in.)
Proud of his clever wordplay as well as his novel idea, Gamow sent a copy of the paper to his friend Swedish physicist Oskar Klein, advising him of its importance. “It seems that this ‘alphabetical’ article may represent alpha to omega of the element production,” Gamow wrote. “How do you like it?” Klein then responded:
“Thank you very much for sending me your charming alphabetical paper. Will you allow me, however, to have some doubt as to its representing ‘the alpha to omega of the element production.’ As far as gamma goes, I agree of course completely with you and that this bright beginning looks most promising indeed, but as to the further development I see difficulties.”
Indeed, Klein’s response was apt. The alpha-beta-gamma paper could literally explain only the first three elements: hydrogen, helium, and (to a limited extent) lithium. These could be built up step by step, like the rungs of a ladder, by adding a proton, neutron, or deuteron (proton-neutron combination) to rise to the next isotope. Beyond lithium production there was a fatal problem: there were no stable isotopes of atomic mass (sum of protons plus neutrons) five or eight!
- Adding either a proton or neutron to helium-4, to create either helium-5 or lithium-5, would cause either one to decay in less than 10–21 seconds.
- Adding two helium-4 nuclei together, to create beryllium-8, results in a decay in just just under 10–16seconds.
Without a good step through mass five or eight, there seemed to be no good way to progress further. There was no way, for instance, carbon could be assembled, especially in the limited time the universe was at its hottest. When you thought about even higher, heavier elements, the problem only grew more difficult. The Big Bang nucleosynthesis ladder was thereby missing key rungs that doomed it as a complete description of the entire periodic table.
Hoyle, in the meanwhile, put forth his own hypothesis that all of the higher elements beyond helium were produced in red giant stars. Over the course of a decade, from the mid-1940s to the mid-1950s, he began to consider various types of nuclear processes that could build up the higher elements, such as carbon, nitrogen, and oxygen in fiery stellar cores. These would require immensely high temperatures sustained for long periods of time.
At Caltech, C.C. (Charles Christian) Lauritsen, a Danish nuclear physicist, had established a powerful nuclear structure center called the W. K. Kellogg Radiation Laboratory. Researchers there in the 1950s included Lauritsen’s graduate student William Fowler, and Lauritsen’s son Thomas, an accomplished physicist in his own right. The lab became distinguished for the use of particle accelerators to speed up and hurl particles toward nuclear targets, causing in some cases transmutations.
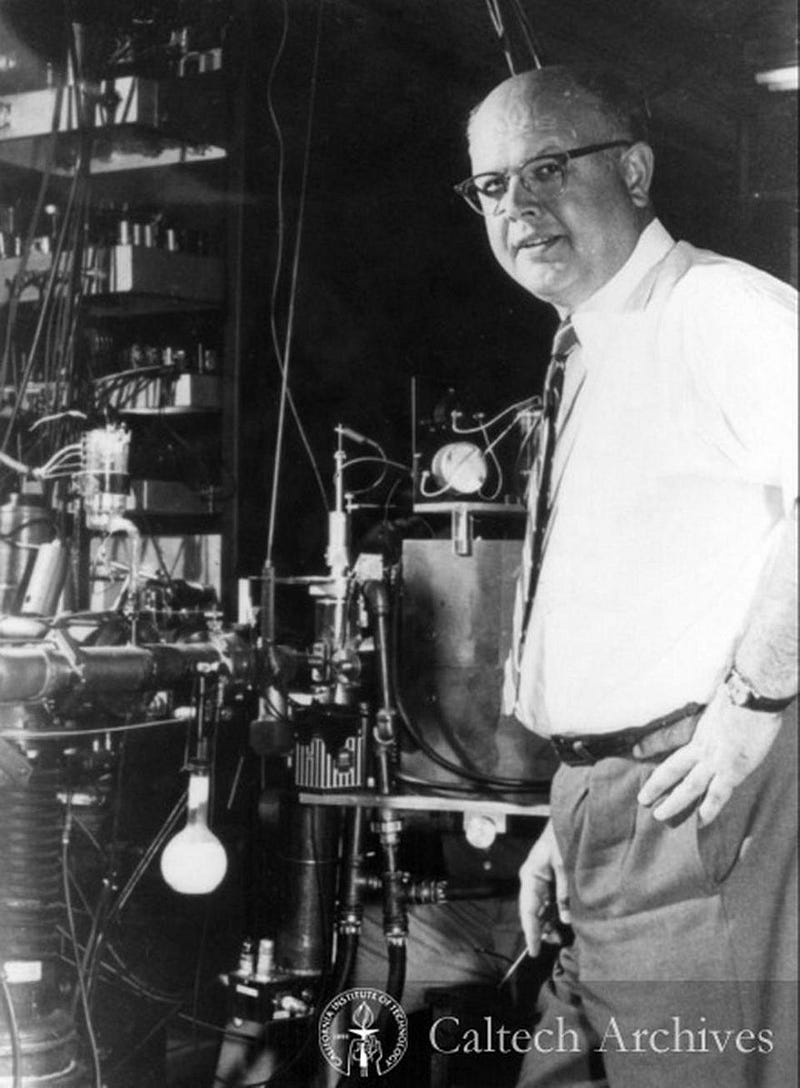
Drawn by the capability of the Kellogg Lab, Hoyle arranged for numerous long visits to Caltech, beginning in 1953. Upon arriving at the lab he immediately challenged the researchers to investigate his hypothesis of a long-lived excited state of carbon-12 that acted as a vital step in stellar nucleosynthesis. Fowler, the two Lauristens, and another physicist named C.W. Cook set out to find that state and managed very shortly to produce it. That began a highly lucrative collaboration between Hoyle, Fowler, and others. They were soon joined by the wife and husband team of British astronomers E. Margaret and Geoffrey Burbidge, who had worked with Hoyle at Cambridge.

On December 30, 1956, the element transmutation work at Kellogg, involving bombarding carbon with deuterons, was featured in the New York Times as evidence for the Steady State theory as opposed to the Big Bang. Referring to a talk delivered by Thomas Lauritsen at the American Physical Society annual meeting that year, its headline read, “Physicist Makes Helium of Carbon; Transmutation is Hailed as Helping to Explain Origin of Universe; ‘Big Bang’ Theory Hit.”

Less than a year later, on October 1, 1957, the two Burbidges, Fowler, and Hoyle (B²FH) published in Reviews of Modern Physics the seminar paper “Synthesis of the Elements in Stars.” Drawing upon Hoyle’s theoretical expertise, the Burbidges’ observational know-how, and Fowler’s experimental prowess (which he picked up in part from C.C. Lauritsen), the paper was a brilliant exposition of how the elements were built up, dividing these into different processes, starting with hydrogen burning and helium burning, and continuing to the so-called “s” (slow neutron capture), “r” (rapid neutron capture) and “p” (proton capture) processes involving higher elements.
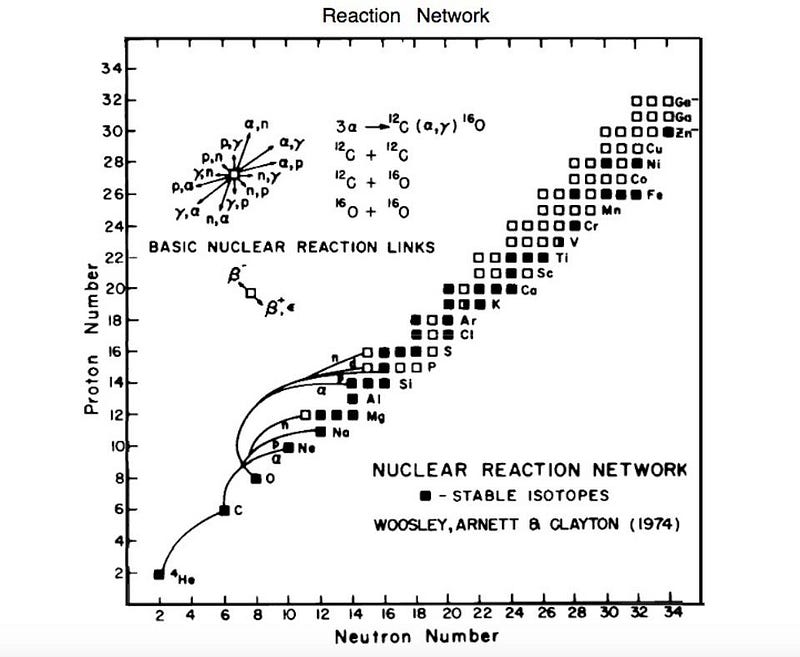
They showed how aging stars that were massive enough, such as Red Giants and Supergiants, could find it energetically feasible to create all the elements up to iron in their cores. The even-higher elements could be produced in the extreme conditions of a supernova explosion, upon which the full gamut of elements would be released into space.
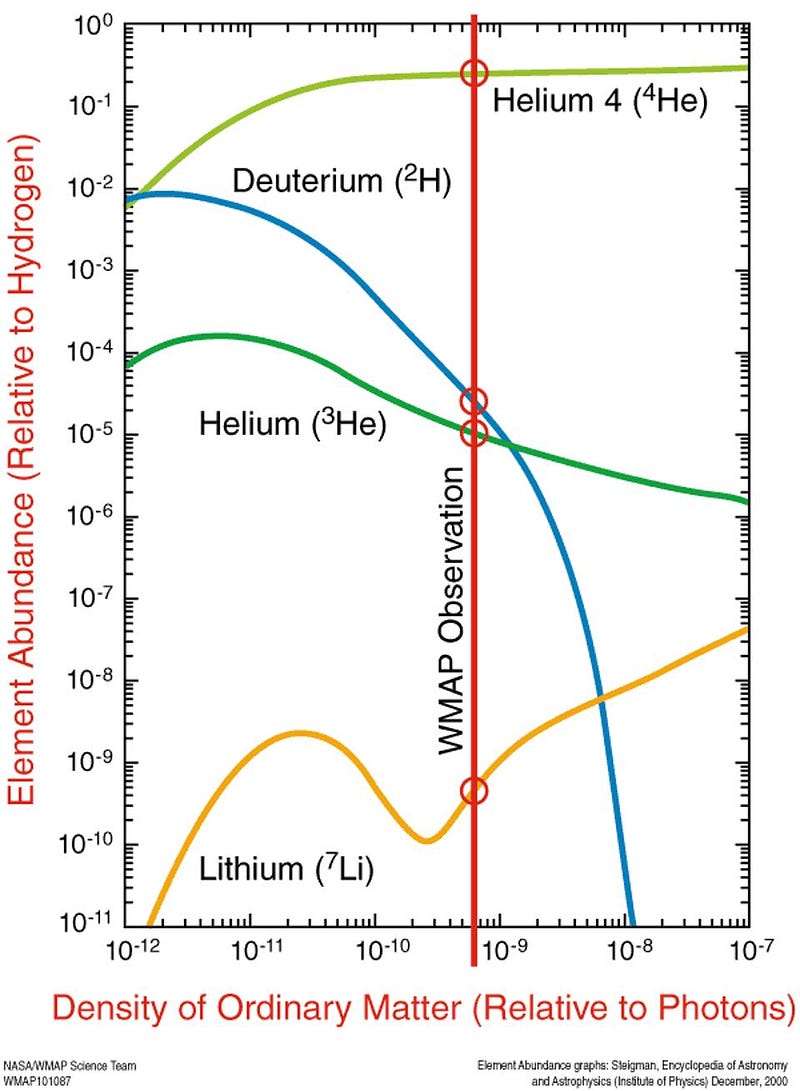
The major limitation of the otherwise outstanding article was its failure to predict the massive amount of helium in space. Although all stars fuse hydrogen into helium, they would only create a Universe that was, by mass, less than 5% helium today. Yet we observe a Universe where more than 25% of its mass is helium. To produce that percentage, the hot Big Bang turned out to be needed. The close match of Big Bang predictions to the actual hydrogen-to-helium ratio, as well as the 1965 discovery by Arno Penzias and Robert Wilson of the cosmic background radiation, the cooled down “hiss” of radiation from the early universe, cemented mainstream astronomers’ support of the Big Bang over the Steady State.
In the mid-1960s, Hoyle and the Burbidges dropped the original Steady State theory, but along with Hoyle’s student Jayant Narlikar developed an alternative with “little bangs” called the quasi-steady state. Until his death in 2001, Hoyle continued to embrace that theory. While Fowler won the Nobel Prize for his nuclear research in general, arguably Hoyle and the Burbidges received relatively little credit for their seminal contributions.
In 2007, along with Virginia Trimble, I helped organize a session at an American Physical Society meeting in honor of the 50th anniversary of the B²FH paper. Geoffrey Burbidge, by then in poor health, aided by a nurse, and confined to a wheelchair, attended and gave a talk. His spirit and voice were as strong as ever, however. I recall him talking about the Big Bang people being like mindless lemmings following their leader over a cliff. He died less than three years later.
Today Margaret Burbidge, at age 97, is the only author of the paper still alive, as we commemorate its 60th anniversary. Let’s raise a toast to Prof. Burbidge and her late colleagues, in celebration of the moment that humankind realized that it is made of star stuff!
Starts With A Bang is based at Forbes, republished on Medium thanks to our Patreon supporters. Order Ethan’s first book, Beyond The Galaxy, and pre-order his next, Treknology: The Science of Star Trek from Tricorders to Warp Drive!





