New drug for fatty liver disease cuts fat by 65%
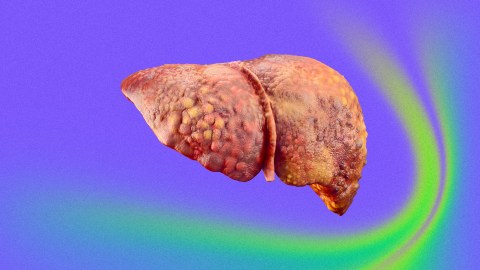
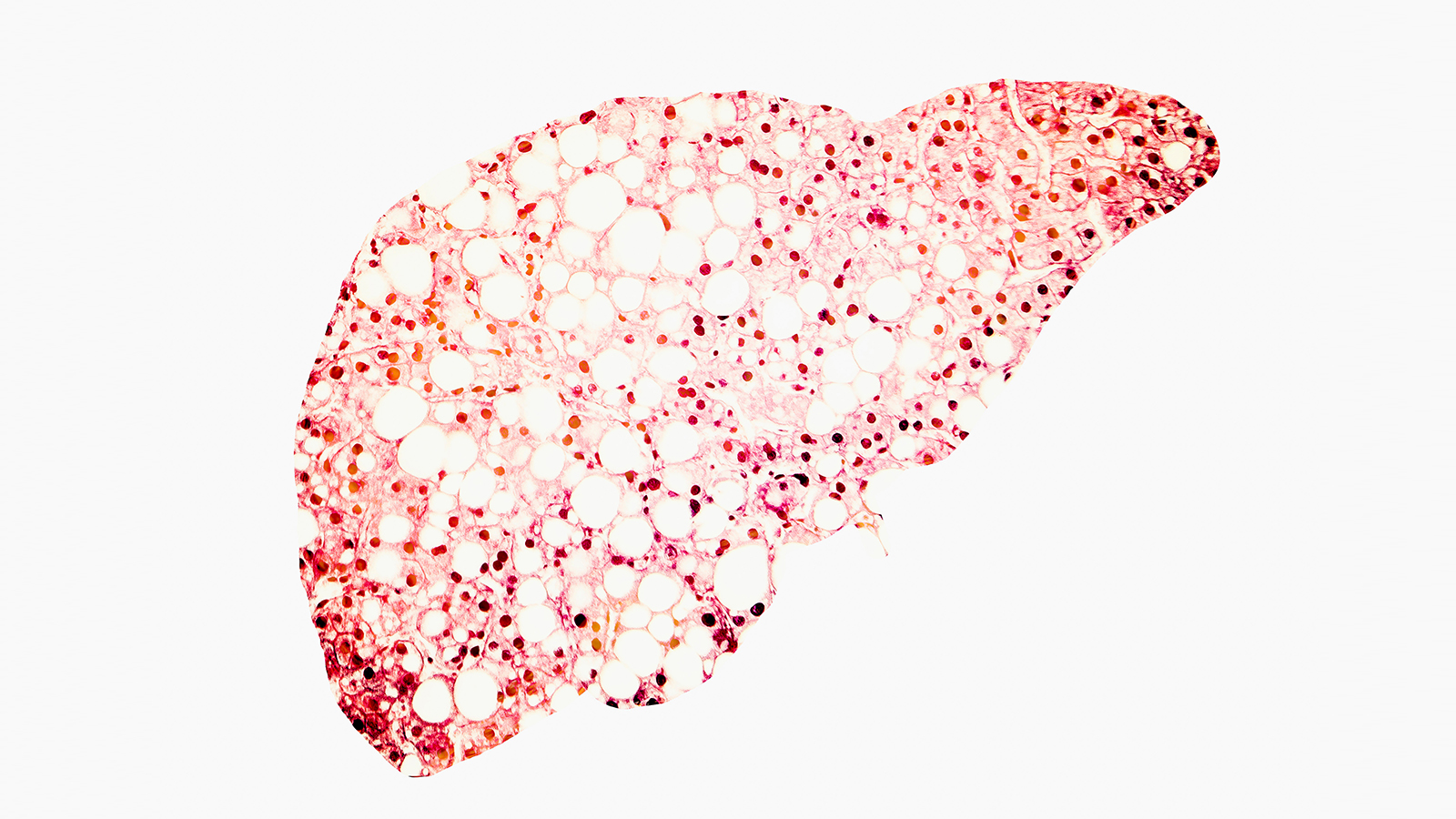
- NASH (non-alcoholic steatohepatitis) is a serious form of fatty liver disease that affects an estimated 2% to 5% of people in the U.S.
- The condition is characterized by a buildup of excess fat in the liver, leading to chronic inflammation and liver damage. It is also an increasingly common reason people require a liver transplant.
- A small trial showed that combination treatment with efruxifermin and a GLP-1 agonist can reduce liver fat by 65%.
A new treatment for an advanced form of fatty liver disease appears not only to work, but also be compatible with drugs for obesity, diabetes, and related health issues.
The challenge: NASH (non-alcoholic steatohepatitis) is a serious form of fatty liver disease. It affects an estimated 2% to 5% of people in the U.S. and is characterized by a buildup of excess fat in the liver, leading to chronic inflammation and liver damage.
NASH is an increasingly common reason people require a liver transplant in the U.S.
Several common conditions raise the risk of NASH, including obesity, diabetes, high cholesterol and triglycerides, and metabolic syndrome; genes and the microbiome may play a role as well.
Unchecked, NASH can cause cirrhosis, liver failure, and cancer, and it’s an increasingly common reason people require a liver transplant in the U.S. Improved diet and increased exercise can slow or even reverse its progression, but there’s currently no approved medical treatment.
NASH treatment: Some researchers believe a class of drugs called “GLP-1 agonists” will be able to treat NASH. Semaglutide (sold as Ozempic and Wegovy) is one of the most well-known of these meds, and it’s already being used to treat obesity and diabetes, two significant risk factors for NASH.
A small study by biotech company Akero Therapeutics suggests that these drugs might not be able to treat NASH on their own — but they do seem to help if combined with efruxifermin (EFX), Akero’s in-development NASH treatment.
The trial: Akero’s 12-week study involved 31 patients with type 2 diabetes and liver scarring due to NASH. All of the participants were already taking a GLP-1 drug to treat diabetes or obesity, and for the duration of the study, 21 of them began taking EFX, too. The other 10 took a placebo.
“We can assume that GLP-1 will be very widely used in this population.”
TIM ROLPH
At the end of the study, patients in the EFX group had 65% less liver fat, on average, than when they started, and 88% of them ended up with what’s considered a normal amount of fat in their livers. In the placebo group, the average reduction in liver fat was 10%, and by the end of the 12 weeks, just 10% of participants had normal liver fat levels.
The combination NASH treatment appeared to be well tolerated as well, with mild gastrointestinal issues being the most common adverse events. One participant dropped out of the study due to nausea, but no serious drug-related adverse events were reported.
“We can assume that GLP-1 will be very widely used in this population, even without having a formal NASH label,” Tim Rolph, Akero’s co-founder and CSO, told STAT News. “So being compatible, if you will, and bringing additional value specific to NASH on top of these therapies, I think, is a profile that will enable us to be successful.”
The combination treatment reduced liver fat by 65% compared to 10% for a GLP-1 alone.
Looking ahead: This was a very small study, so more research is needed to find out whether EFX is an effective NASH treatment, with or without the addition of a GLP-1 drug.
Akero expects to report the results of a larger phase 2B trial of only EFX by the end of 2023, and it also plans to launch two phase 3 trials of the drug this year.
“With the added support of this newest data set, we believe EFX has the potential to play an important role in treating patients with NASH who are receiving GLP-1 therapy in addition to the potential to be a foundational monotherapy for patients with NASH,” said Kitty Yale, Akero’s chief development officer.
This article was originally published by our sister site, Freethink.