What Was It Like When The Universe Made Its First Elements?

Before there were humans, planets, or even stars and galaxies, we had to make the first elements. Here’s how they happened.
From the first moments of Big Bang to the present day, the cosmic story of how our Universe evolved to become filled with stars, galaxies, and all that we can see and detect is a tale that unites us all. Although we began in an incredibly hot and dense state, the Universe expanded. That expansion spreads everything in the Universe out, reduces its energy and temperature, and compels particles to interact, decay, and freeze out.
By time the Universe is 3 seconds old, there are no more free quarks; there is no more antimatter; neutrinos no longer collide with or interact with any of the remaining particles. We have more matter than antimatter, more than a billion photons for every proton or neutron, and the Universe is a little under 10 billion K in temperature. But it cannot yet make elements. Here’s how that step happens.
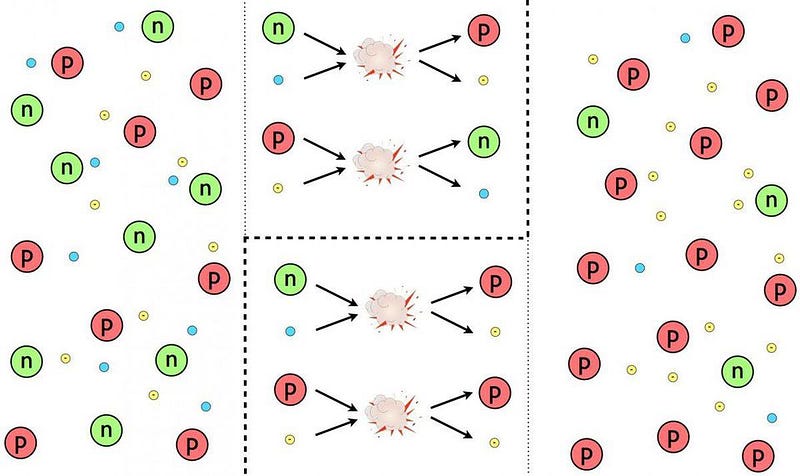
A whole slew of things happened in the first 3 seconds of the Universe, but one of the last things to happen is most important for what comes next. The Universe was filled with protons and neutrons, which would — at high enough energies — collide with electrons or neutrinos to interconvert, or switch from one type to the other. The reactions all conserved baryon number (the total number of protons and neutrons) and electric charge, meaning that this phase began with a 50/50 split between protons and neutrons, with just enough electrons to balance the number of protons.
But because the neutron is more massive than the proton. It needs more energy via Einstein’s E = mc² to be created from a proton than vice versa. As the Universe cools, more neutrons turn into protons than the other way around. By time all is said and done, the Universe is 85–86% protons (with an equal number of electrons) and just 14–15% neutrons.
With protons, neutrons, and electrons all flying around under extremely hot, dense conditions, you might think it would lead to something like what’s going on in the center of our Sun. It would be so reasonable to think about protons and neutrons fusing together, building up heavier and heavier elements as they climb the periodic table, and giving off energy via Einstein’s E = mc², as these reactions must inevitably do. After that, electrons would bind to those nuclei, producing the full gamut of stable, neutral elements found in the periodic table today.
These are the elements we see, after all, in the Sun and all stars. They had to come from somewhere, right?
The odd thing is this: the elements do come from somewhere, but not from the Big Bang. No less an authority than George Gamow — the founder of the Big Bang theory — claimed that this hot, dense crucible was the perfect spot to form these elements. Gamow was mistaken, however. The Universe does form elements during the hot Big Bang, but only a very select few.
There’s a reason for this that Gamow never anticipated, and that most of us may not have thought of at first glance, either. You see, in order to make elements, you need enough energy to fuse them together. But in order to keep them around and build heavier things out of them, you must make sure you don’t destroy them. And this is where the early Universe lets us down.
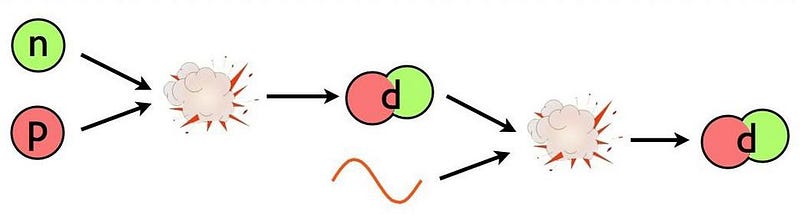
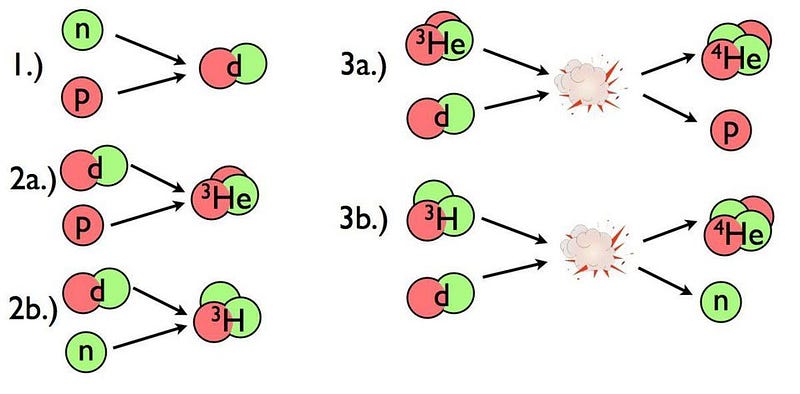
At three seconds of age, let’s say the Universe is filled with 85% protons (and an equal number of electrons), 15% neutrons, and about 1-to-2 billion photons for every proton or neutron. In order to build a heavy element, the first step must be to either collide a proton with a neutron or a proton with another proton. The first step towards building anything more complicated out of the basic building blocks of atoms is to create a nucleus with two nucleons (like a proton and neutron) bound together.
This part is easy! The Universe makes deuterium nuclei, abundantly, with no problem. The problem is that the instant we make it, it’s immediately destroyed.
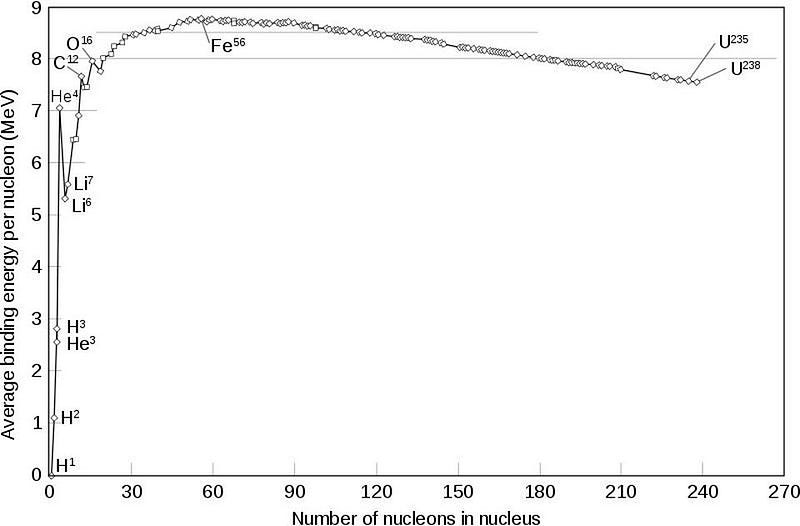
In a hot, dense Universe where photons far outnumber protons and neutrons, the overwhelming odds are that the very next thing to collide with your deuteron will be a photon. (The odds are less than 1-in-a-billion that it won’t be a photon!) And at these energies, those photons have more than enough energy to immediately blast that deuteron back apart into a proton and neutron. Even though a deuteron is less massive by about 2.2 MeV (mega-electron volts) than an individual, free proton or neutron, the photons are energetic enough to more than make up for that mass difference. Unfortunately for the Universe, Einstein’s E = mc² can prevent you from building what you want, too.
Deuterium is constantly being created; but just as quickly as we can make it, it’s being destroyed. And without that first step on our elemental staircase in place, we can go no farther. As long as the Universe is this hot, there is nothing we can do but wait. This is why cosmologists call this time in the Universe the deuterium bottleneck: we would love to build heavier elements and we have the material to do so, but we must pass through this easily-destroyed deuterium step, and cannot. At least, not yet.
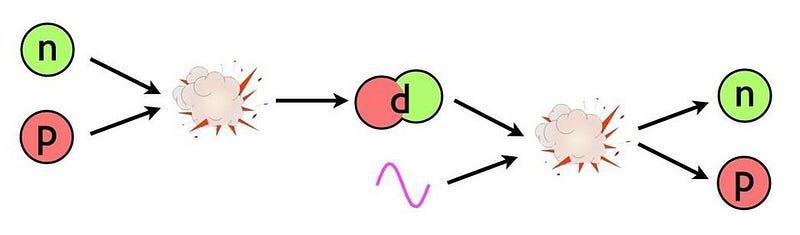
So we wait. We wait for the Universe to cool, which means it has to expand, stretching the photons’ wavelengths, until they fall below the threshold to break deuterium apart. But this takes more than three minutes to happen, and in the meantime, something else takes place. The unbound neutrons, so long as they’re free, are unstable, and start to decay.
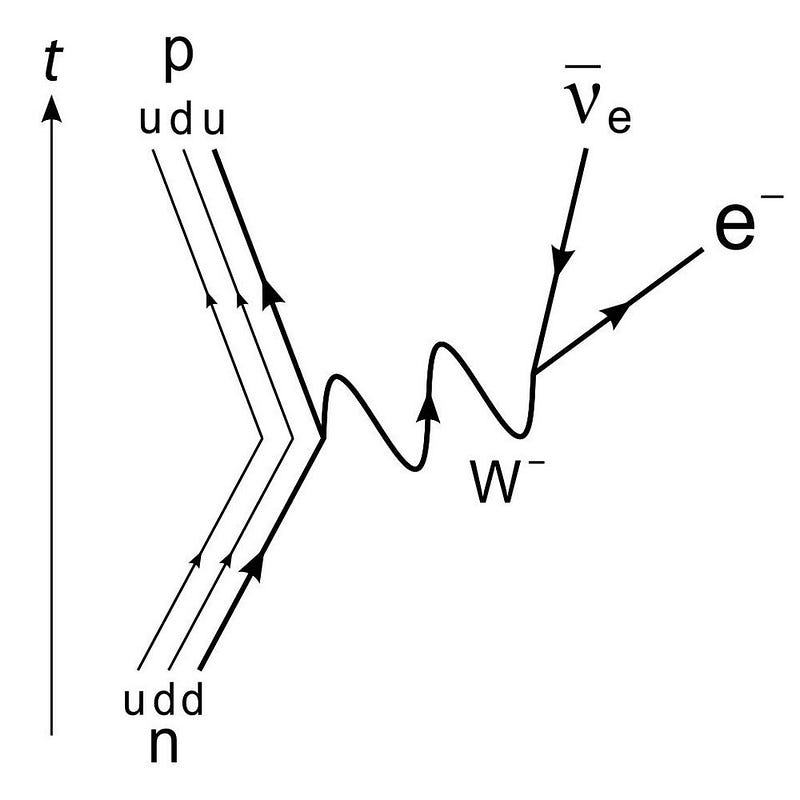
A free neutron has a half-life of about 10.3 minutes. This means that if we wait around long enough, every neutron that we have will decay into a proton, an electron, and an anti-electron neutrino. In terms of an equation, it would look like this:
- n → p + e- + anti-νe
The actual time it takes for the Universe to expand-and-cool to the point where deuterium isn’t immediately blasted apart is about 3.5 minutes, meaning that about 20% of the neutrons decay into protons over this timespan. What was a 50/50 split between protons and neutrons in the early stages became an 85/15 split after 3 seconds, and has now, after more than three minutes, become 88% protons and 12% neutrons.
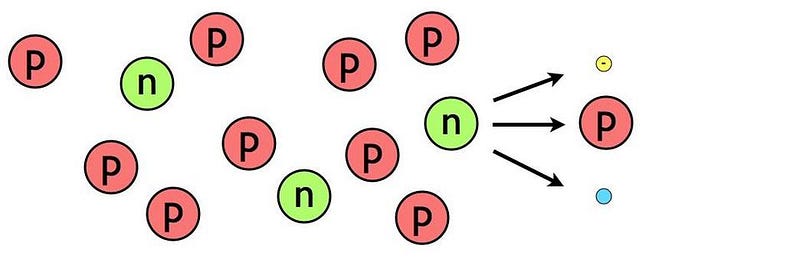
But now the fun starts. At last, the Universe is cool enough that we can not only build deuterium, but build up and up the periodic table from there. Add another proton to a deuteron and you get helium-3; add another neutron to a deuteron and you get hydrogen-3, better known as tritium. If you then add a deuteron to either helium-3 or tritium, you get helium-4 out, plus either a proton or neutron, respectively. By time the Universe is 3 minutes and 45 seconds old, practically all of the neutrons have been used to form helium-4.
The Universe, by mass, is now:
- 76% hydrogen (protons),
- 24% helium-4 (2 protons and 2 neutrons),
- 0.01% deuterium (1 proton and 1 neutron),
- 0.003% tritium and helium-3 combined (tritium is unstable and will decay to helium-3, with 2 protons and 1 neutron), and
- 0.00000006% lithium-7 and beryllium-7 (3/4 protons and 4/3 neutrons, formed from tritium/helium-3 and helium-4 fusing together).
The big problem is that by this time, the Universe has expanded and cooled enough that its density is only one-billionth the density in the Sun’s core. Nuclear fusion cannot occur any longer, and there are no ways to stable fuse either a proton with helium-4 or two helium-4 nuclei. Li-5 and Be-8 are both highly unstable, and decay away after a tiny fraction of a second.
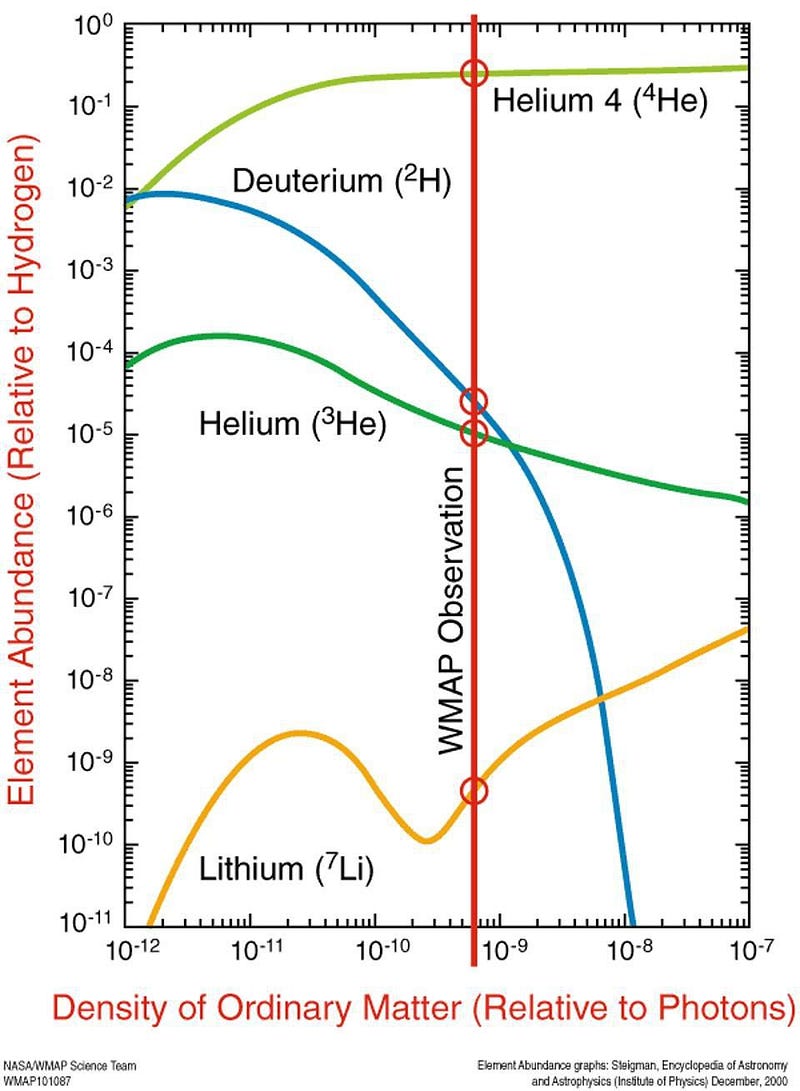
The Universe does form elements immediately after the Big Bang, but almost all of what it forms is either hydrogen or helium. There’s a tiny, tiny amount of lithium left over from the Big Bang, since beryllium-7 decays into lithium, but it’s less than 1-part-in-a-billion by mass. When the Universe cools down enough that electrons can bind to these nuclei, we’ll have our first elements: the ingredients that the very first generations of stars will be made out of.
But they won’t be made out of the elements we think of as essential to existence, including carbon, nitrogen, oxygen, silicon and more. Instead, it’s just hydrogen and helium, to the 99.9999999% level. It took less than four minutes to go from the start of the hot Big Bang to the first stable atomic nuclei, all amidst a bath of hot, dense, expanding-and-cooling radiation. The cosmic story that would lead to us has, in truth, finally begun.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.





