Mini-Movie Monday: Genesis Episode 4: Atoms
The early Universe consisted of atoms, but 99.999999% of them were Hydrogen and Helium. Where’d the rest come from?
“A physicist is just an atom’s way of looking at itself.” –Niels Bohr
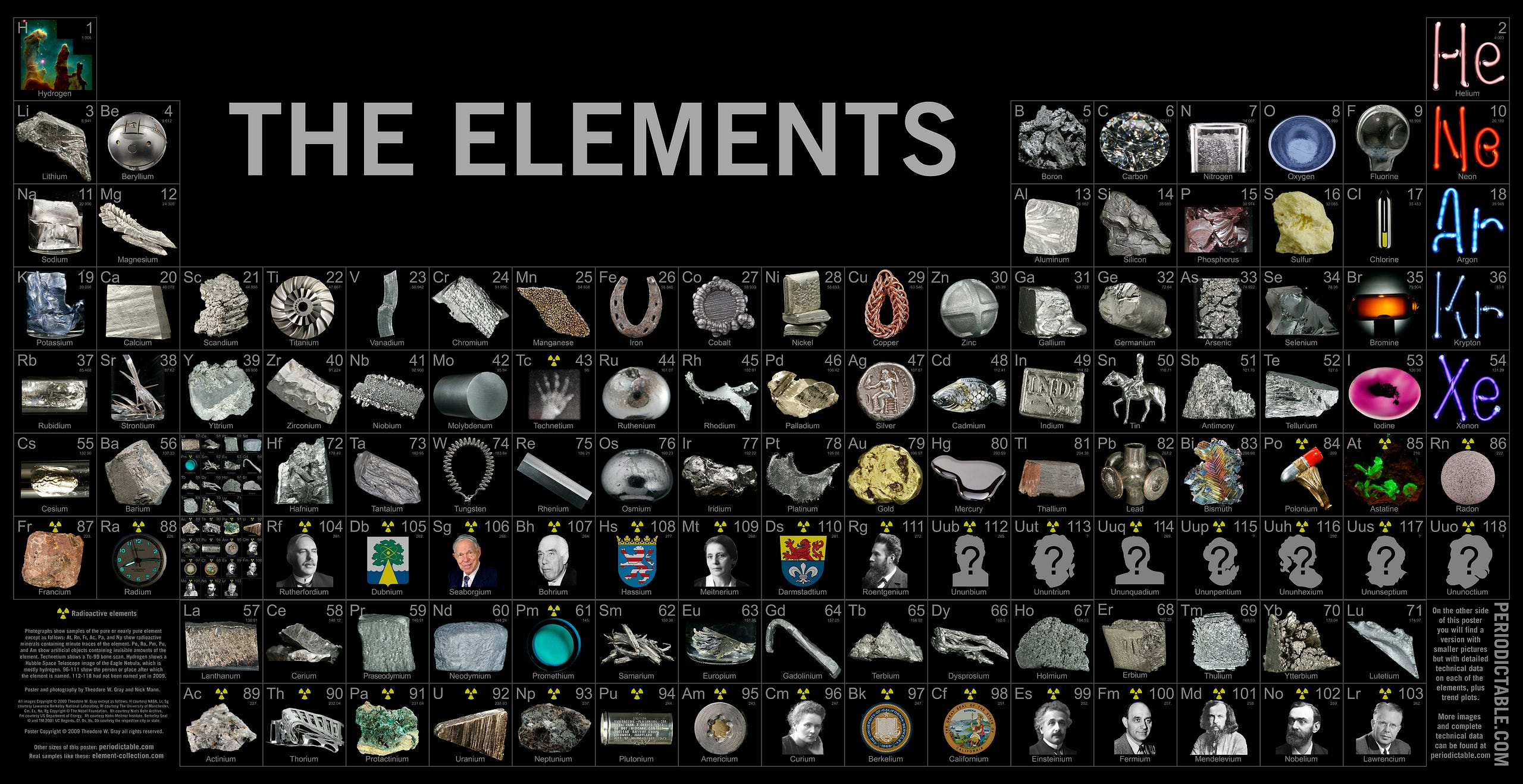
In the story of where all this comes from, atoms are perhaps the nearest and dearest component of that. Without them, none of the structures that we know of would exist, from molecules to cells to humans to planets and stars. Yet we’ve been able to discover more than 100 different types of them, some 90 or so of which occur naturally here on Earth.
So how did all these atoms come to be? Enjoy Genesis, Episode 4: Atoms, and if you like, read the full transcript below!
As human beings in the world, we have the unique capacity among all other living things to understand, at a fundamental level, exactly what it is that makes us up. Our bodies aren’t just made of organs or even cells, but of atoms.
Down at a scale ten billion times smaller than a human being, negatively charged electrons orbit a positively charged nucleus, with nature giving us more than 90 different naturally occurring varieties. This is actually vital for us, since different combinations of atoms can bind together in different ways, giving rise to a seemingly endless variety of chemical and biological permutations.
But for all the different ways atoms come together in this world today, the possibilities were incredibly limited back in the early Universe, shortly after the Big Bang. For the first few million years of the Universe, hydrogen and helium were virtually all that existed as far as atoms went. So where did the ingredients that we needed in order for our world to exist come from? To answer that, we need to head back in time, to stars that formed many billions of years ago.
When massive, dense regions of neutral atoms grow to a sufficient size, they contract and collapse under their own gravity, giving rise to regions where nuclear fusion can ignite, leading to the first generation of stars.
Deep in their cores, hydrogen fuses into helium, with heavier elements to follow, building up to higher and higher numbers in the periodic table in a series of chain reactions. While most of the stars will be small and long-lived, the brightest, bluest stars will burn through their fuel incredibly quickly, hitting a limit where no more energy can be gained by burning elements like iron, nickel and cobalt.
When the core runs out of burnable fuel, the interior implodes to form either a neutron star or a black hole, while the outer layers — full of heavy elements that can become even heavier by capturing neutrons — get returned to the interstellar medium of the galaxy, where they’ll be incorporated into future generations of stars. Other, less massive stars get in on the action, too, by blowing off their outer layers as well, further adding large amounts of essential elements for life like carbon, nitrogen and oxygen, while their interiors collapse down to a white dwarf star.
Not only do the outer layers of massive stars come to enrich the interstellar medium, but the inner cores have opportunities to merge together with similar objects, triggering runaway fusion reactions that create tremendous amounts of heavier elements, including some of the most precious elements on Earth, like titanium, silver, palladium, gold and platinum.
All the elements heavier than helium — including more than 90% of your body, by mass — owe their origins to generations of stars that existed long before our solar system ever formed.
After billions of years of cosmic evolution, here we are today. It’s amazing what the Universe can do with just two ingredients: the lightest elements of all, and time.
Catch the previous episodes on Organic Molecules, our Solar System, and the Galaxy. And leave your comments on the Starts With A Bang forum here!





