The slow dance that made you
We owe our origins to the stars. But it’s not the fast catastrophes that made us possible, but a slow, burning romance.
“It took less than an hour to make the atoms, a few hundred million years to make the stars and planets, but five billion years to make man!” –George Gamow
When you think about where we came from, you probably think of the terrestrial, recent story of us. Maybe you think of your parents and their parents and so on, which is certainly a part of it. Maybe you think of all the animals that came before, and the evolutionary twists and turns that brought you here. Or maybe you go back even farther, and think of the very elements themselves that the Earth is made out of.

It’s these, after all, that enabled us to exist at all. Without the different elements — and all the different molecular combinations that they can form — there would certainly be no story of us.
Yet when we look at the periodic table of elements, some ninety-ish of which occur naturally here on Earth, it’s hard to help but wonder at where they came from.

Sure, we can give you the quick answer and say “from prior generations of stars.” While this is surely true, it’s hardly satisfying. After all, stars come in many different varieties, which live and die either slowly or rapidly, depending on what type of star they are.

Whenever we form stars, we do so in bunches: clusters of hundreds, thousands, or all the way up to many millions of stars all at once. Sure, if you look at any one of them, you’re likely to notice the brightest, bluest ones, since they’re the easiest to see and the most prominent. These stars are also the shortest-lived, since they burn through their fuel the fastest and shine so incredibly brightly: up to tens of thousands of times brighter than our own Sun!

What happens inside these stars, the brightest, most massive ones? Just like all stars, they begin by burning hydrogen into helium: the two most abundant elements in the Universe. When they run out of hydrogen in their cores, the massive, helium-filled region starts to contract, as there’s no more pressure from nuclear fusion to hold the star up against gravitation.
But as it contracts, it also heats up. In stars that are massive enough (and this will include our Sun, in time), helium will begin fusing into carbon as well. And while our Sun won’t be able to fuse carbon into heavier elements, stars that are four-to-eight times more massive than our own do. And they form oxygen, and then silicon and sulfur, and then iron, nickel and cobalt.

This process happens rapidly, however, and so while it leaves you with lots of oxygen and silicon, a large abundance of sulphur, and quite a bit of iron/nickel/cobalt, it doesn’t have a lot of time to build up a diversity of elements.
Sure you can get some of the ultra-heavy ones, and small amounts of the others in the periodic table when the star goes supernova!

The collapse of the inner core leads to the spontaneous production of neutrons, which collide with all the surrounding elements to bump them up the periodic table in a rapid chain reaction known (completely uncreatively) as the r-process, where r stands for rapid.
But this process isn’t nearly enough to explain most of the interesting elements we see here on Earth. And the elements on Earth are interesting.

Moreover, they don’t seem to line up with what we expect to be formed from these most massive of stars. What’s with all the aluminum, for example? Why the roughly even distribution of all these elements up the periodic table?
As it turns out, while practically all the elements on our planet were once inside a star that went supernova, most of them went through more than one star.

In a star like our Sun — one that won’t go supernova — when it reaches the end of its life, it expels its outer layers in a planetary nebula, returning that material to the interstellar medium. As you can see in the (false-colored) images above, this includes a huge variety of elements, where each color indicates the signature of a different member of the periodic table.
But what might surprise you is that it’s actually the quiet, normal life of stars like our Sun that gives rise to the elements that are so familiar to us!
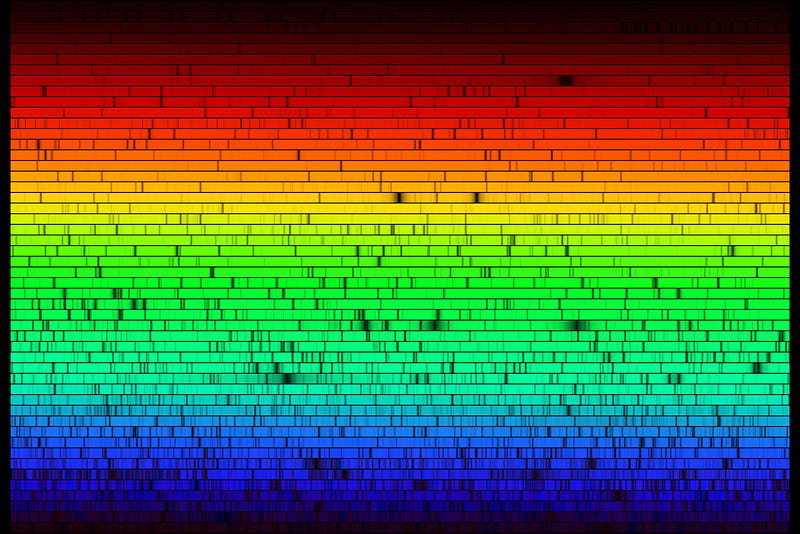
Take a look at the solar spectrum: all the different absorption lines from the different elements in the Sun. What might surprise you is that one of the elements we find in the Sun is the element Technetium, an element with no stable isotopes, and that was never found naturally occurring here on Earth.

But it is in the Sun! How does this happen?
There’s a slower, steadier process that forms elements in stars like the Sun: the (also boringly named) s-process, where the s stands for slow. So long as you have elements like carbon and neon in your star, you’re going to make neutrons. When a helium nucleus collides with carbon-13 (a stable, but less-common isotope of carbon than the normal carbon-12), it fuses into oxygen, but also releases a free neutron. Similarly, when a helium nucleus collides with neon-22 (again, a common, stable isotope of neon making up about 9% of all the neon on Earth), it fuses into magnesium-25, also emitting a free neutron.
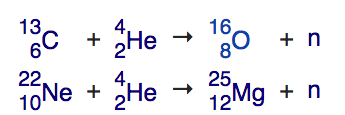
These neutrons — like all free neutrons — are special. Without having a charge to them, it’s easy for them to run into other nuclei inside a star, where they can be absorbed, helping build heavier elements out of lighter ones. But they also have a time limit: free neutrons only live about 15 minutes, on average, before decaying away into protons and lighter particles.
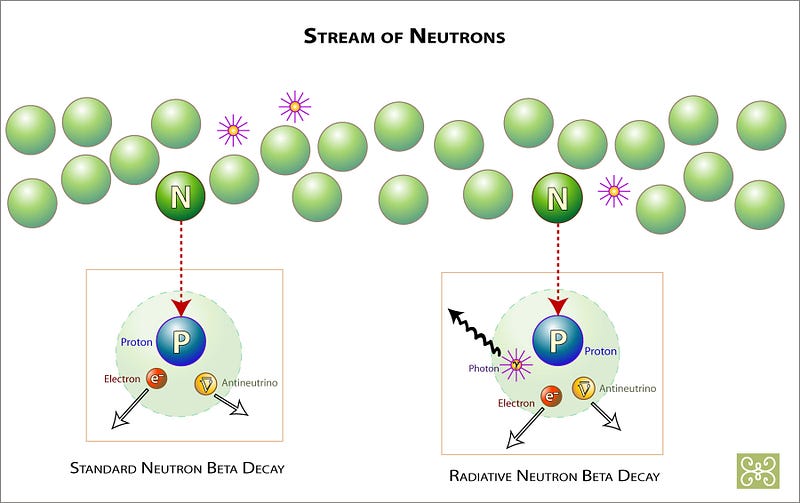
So you need to run into something quickly enough to produce a heavier element, which is why you form them most efficiently if you’re inside a star! This is not only how you get Technetium, but also many of the elements that are the most common in life processes here on Earth, including:
- phosphorous,
- sodium,
- chlorine,
- magnesium,
- calcium,
- potassium,
- copper, and
- zinc.
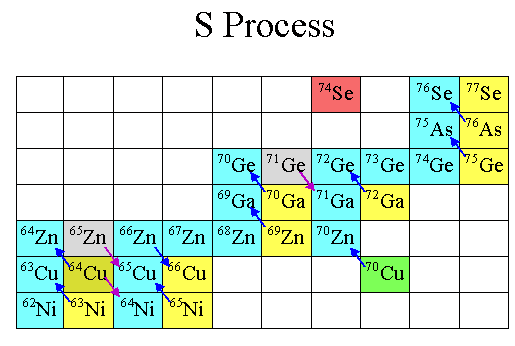
The chain reaction is simple: you keep adding neutrons to climb to higher and higher isotopes, until one is unstable and decays to the next element up on the periodic table. Then, you add more neutrons, and the process repeats.
In fact, if you look at the color-coded periodic table, below, you’ll find that every element with a green “L” around it is one that’s primarily produced in the Universe by this mechanism of slow neutron capture.
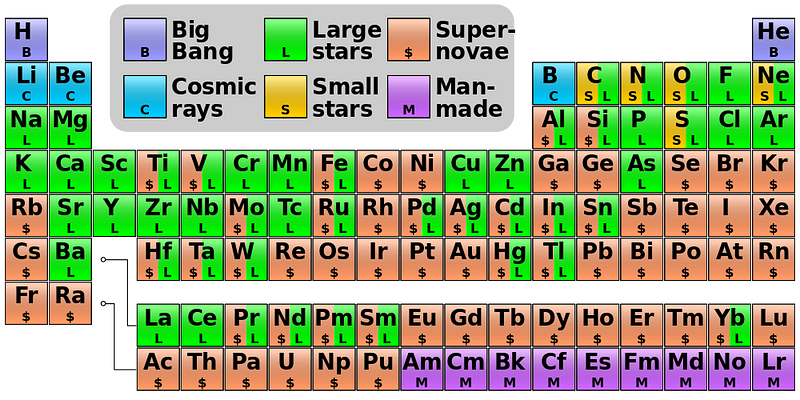
You can get all the way up to lead through the s-process simply by starting with iron, but if you try adding neutrons to it, you’ll produce a little bit of Bismuth, but it will decay back down to lighter elements. There’s no going up past that point without a supernova.
Nevertheless, it’s this slow, long-lasting, perhaps romantic process that’s enabled the elements that we require to exist. Deep within the hearts of stars, at millions of degrees, helium nuclei are running into these uncommon but stable isotopes that were formed in prior generations of stars, producing free neutrons and slowly building a huge variety of elements out of what were initially “boring” things like oxygen, silicon, sulphur and iron/cobalt/nickel.

So when you think of the elements that make life possible, and the fact that we owe our origins to the stars, don’t just think of the spectacular, flashy supernovae. The story is so much richer than that, and requires a slow-burning fire to give rise to us. In the end, we owe our very existence to the relentless furnace of the s-process.
Leave your comments at the Starts With A Bang forum on Scienceblogs!





