We exist thanks to chirality and the asymmetry of life

- Life on Earth is fundamentally asymmetric.
- As Louis Pasteur discovered in the mid-1800s, the amino acids and sugars that make up living matter display a fundamental asymmetry: Life prefers “left-handed” amino acids and “right-handed” sugars.
- The resolution of this mystery will be essential for understanding the origin of life here and elsewhere in the Universe.
Last week, we discussed the fundamental cosmic asymmetry between matter and antimatter, and why it is essential for us being here. To recall the main lesson, if matter and antimatter existed in equal amounts in the Universe, they would mostly annihilate by colliding with each other, leaving behind a Universe filled mostly with radiation — boring photons streaming across space. No stars, no galaxies, and, of course, no planets or life. There is a beautiful line connecting this imperfection at the smallest possible scales — that of the elementary particles of matter — to life itself.
This connection forces a rethinking of the complementary role of symmetry and asymmetry in the Universe. They are both essential. To adopt an aesthetic of Nature as being limited to perfection — or, to recall Keats, to associate beauty with truth — leads to a biased and incomplete view of the reality of existence.
The asymmetry of life
Today, we follow our previous discussion of asymmetry in particle physics with the asymmetry in life. The origin of life is a complex, multifaceted question. Imperfections played a central role in this story, a story that deserves several essays. Here, we will focus only on the molecular asymmetry of life.
First, we note that life is an imbalance that recreates itself. At the most fundamental level, life is a complex network of biochemical reactions capable of metabolizing energy and of reproducing following Darwinian evolution. Reactions are a mechanism for a system to reach a certain level of balance or equilibrium. Imbalance leads to change that leads back to balance. At the genetic level, mutations are essential for diversity. The incredible variety of living creatures ultimately springs from imperfections at the reproductive level.
The asymmetry of life starts at the molecular level. For this, we can thank Louis Pasteur (who is famous for discovering that fermentation, such as that occurring in the making of wine and beer, is a biological process due to the presence of microorganisms). In 1849, the 26-year-old Pasteur was working toward his PhD at the École Normale Supérieure in Paris, eager to make his mark among French chemists.
His studies concerned the properties of tartaric acid, a crystalline organic acid present in unripe grapes. Tartaric acid can also be produced in the laboratory through chemical synthesis. Pasteur knew that the acid extracted from grapes and the one produced in the lab had different optical properties — that is, they interacted differently with light. Hidden in this fact is a remarkable property of life, perhaps the key to life itself. To fully appreciate this, a brief physics lesson about light is necessary.
Pasteur sees the light
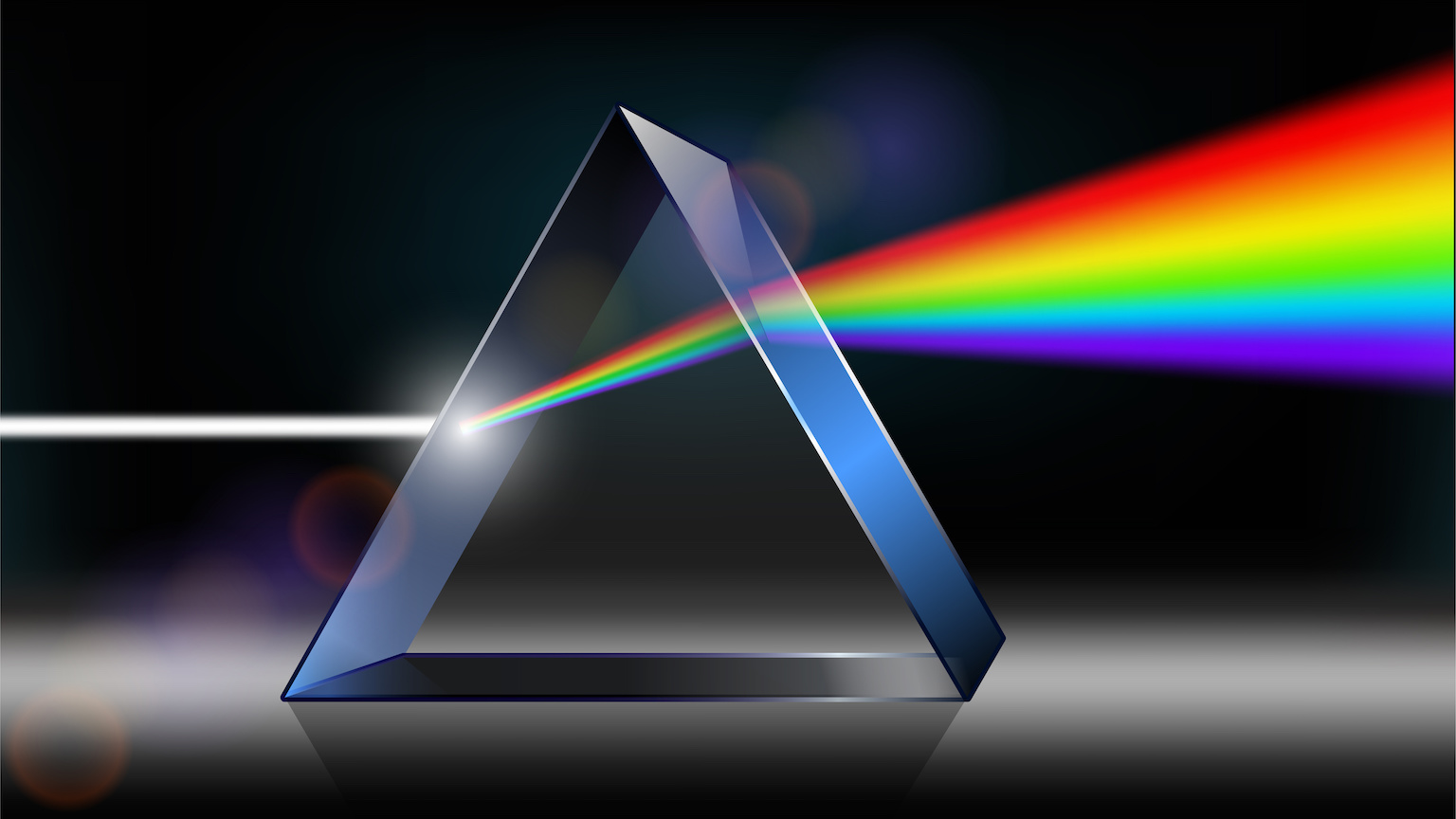
Typically, light waves travel as perpendicular electric and magnetic fields that rotate like propeller blades. Polarized light doesn’t rotate; instead, its electromagnetic fields are confined to a single direction of oscillation. This is akin to a propeller that isn’t rotating. Using this analogy, “rotation of plane polarized light” (see image) simply means a turn to the left or to the right of the “blades” by a particular angle.
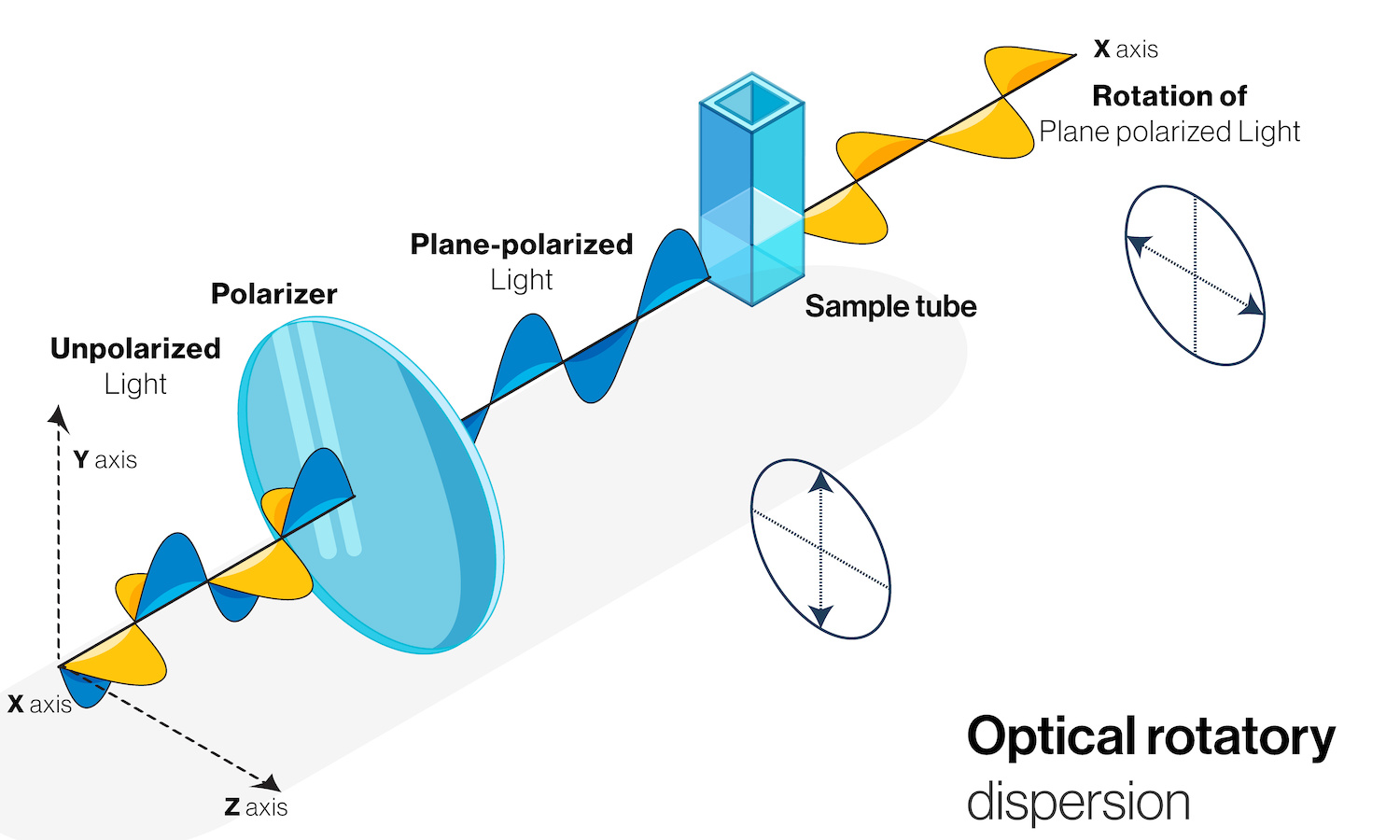
In 1815, the French physicist and chemist Jean-Baptiste Biot discovered that when light travelled through liquid solutions composed of several naturally occurring organic products, its polarization was affected. In the “propeller” analogy, these substances could turn the blades of the propeller (light’s direction of polarization) either to the left or to the right.
Building upon Biot’s research, Pasteur established that when linearly polarized light passed through a solution of tartaric acid synthesized in the lab, nothing happened: the synthetic solution was optically inactive. But when polarized light passed through a solution containing acid extracted from grapes, and thus from a living entity, its polarization direction changed (that is, the blades of the propeller turned a bit). Pasteur realized that since both substances had identical chemical properties, their molecules had the same types of atoms. What then could cause such puzzling asymmetric optical behavior? Could molecules belonging to living and nonliving matter, even if apparently identical, have different properties?
Pasteur examined the crystals from both substances under a microscope. He noted that whereas the lab-synthesized acid had two kinds of crystals, the acid from grapes had only one. With tremendous patience, he separated samples of both crystals using tweezers. Passing light through two solutions made with each of the two types, he demonstrated that the different crystals rotated the polarization plane of light in opposite directions, one to the left and the other to the right. Thus, he discovered that naturally occurring biomolecules had only one type of crystal and hence rotated light only in one direction. Lab-made biomolecules featured both types of crystals and hence rotated light in both directions. But, the net result of that is no rotation at all.
Chirality: mirror-image molecules
Pasteur went on to suggest that there are two kinds of molecules in nature: those that, like water, occur only in one spatial conformation, and those that, like tartaric acid, can occur in two, such that one is the mirror image of the other. The technical term is “chirality,” from the Greek kheir (“hand”). In fact, our hands are an excellent example of chiral asymmetry. Just put one on top of the other. They do not match, which is why you do not want to have two left-handed gloves.
Pasteur expanded his investigation to include several types of organic matter, concluding that there was a fundamental asymmetry in biomolecules that were extracted from living matter, always rotating the polarization of light in the same direction. He famously claimed, L’Univers est dissymétrique! The Universe is asymmetric! We now know that while amino acids, the ingredients that chain up to make proteins, are “left-handed,” sugar molecules are “right-handed.”
Why? Nobody knows, although there are speculations that the split has something to do with the early biochemical development of life. To us, the amazing fact is the existence and persistence of asymmetry in the basic components of all living systems.
Why chirality? Three hypotheses
Although we still do not know how biochirality evolved in early life, there are three interesting possibilities. The first is that it was an accident. As the primordial sludge that became first life evolved in young Earth, life rehearsed many different scenarios, alternating chirality randomly. In a paper with then graduate students Sara Walker and Joel Thorarinson, we developed a model called “Punctuated Chirality,” in which the chiral direction flip-flopped over time until things settled in one direction. (A free version can be found here.) In this scenario, the left-handedness of amino acids was a random accident. Other worlds with life may have right-handed amino acids, a hypothesis we can hopefully confirm fairly soon.
Another possibility is that chirality is influenced by circularly polarized ultraviolet radiation in star-forming regions. UV light has been shown to bias chirality in lab experiments. Planets forming around these stars would be radiated, and life would be skewed in a specific direction. In this case, all planets that happen to have life in this region would share the same chirality. In other regions across the galaxy, chirality could be opposite.
Finally, a third possibility is that chirality could be related to the fundamental asymmetry we find in matter, the left-handedness of neutrinos (or, more precisely, the existence of parity-violating weak neutral currents). Complex calculations do show a small chiral bias, which critics argue is too small to act across the much larger biomolecular scales. However, there could be a yet unknown amplification mechanism that would work at larger scales. In this case, biochirality would be the same across the Universe, such that all amino acids would be “left-handed” and all sugars “right-handed.”
Whatever the resolution of the mystery is, the lesson is that asymmetry plays an essential role in living matter. Why that is so remains an open question — one that, we conjecture, is deeply related to the origin of life itself.